Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis
- PMID: 18376408
- PMCID: PMC2873870
- DOI: 10.1038/nm1738
Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis
Abstract
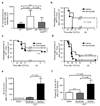
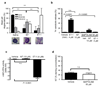
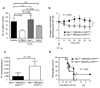
Sepsis is a complex, incompletely understood and often fatal disorder, typically accompanied by hypotension, that is considered to represent a dysregulated host response to infection. Neurotensin (NT) is a 13-amino-acid peptide that, among its multiple effects, induces hypotension. We find that intraperitoneal and plasma concentrations of NT are increased in mice after severe cecal ligation and puncture (CLP), a model of sepsis, and that mice treated with a pharmacological antagonist of NT, or NT-deficient mice, show reduced mortality during severe CLP. In mice, mast cells can degrade NT and reduce NT-induced hypotension and CLP-associated mortality, and optimal expression of these effects requires mast cell expression of neurotensin receptor 1 and neurolysin. These findings show that NT contributes to sepsis-related mortality in mice during severe CLP and that mast cells can lower NT concentrations, and suggest that mast cell-dependent reduction in NT levels contributes to the ability of mast cells to enhance survival after CLP.
Figures



References
-
- Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116. - PubMed
-
- Grocott-Mason RM, Shah AM. Cardiac dysfunction in sepsis: new theories and clinical implications. Intensive Care Med. 1998;24:286–295. - PubMed
-
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

