A substrate in pieces: allosteric activation of glycerol 3-phosphate dehydrogenase (NAD+) by phosphite dianion
- PMID: 18376850
- PMCID: PMC2752152
- DOI: 10.1021/bi8001743
A substrate in pieces: allosteric activation of glycerol 3-phosphate dehydrogenase (NAD+) by phosphite dianion
Abstract
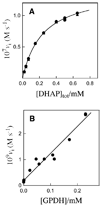
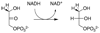
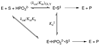
The ratio of the second-order rate constants for reduction of dihydroxyacetone phosphate (DHAP) and of the neutral truncated substrate glycolaldehyde (GLY) by glycerol 3-phosphate dehydrogenase (NAD (+), GPDH) saturated with NADH is (1.0 x 10 (6) M (-1) s (-1))/(8.7 x 10 (-3) M (-1) s (-1)) = 1.1 x 10 (8), which was used to calculate an intrinsic phosphate binding energy of at least 11.0 kcal/mol. Phosphite dianion binds very weakly to GPDH ( K d > 0.1 M), but the bound dianion strongly activates GLY toward enzyme-catalyzed reduction by NADH. Thus, the large intrinsic phosphite binding energy is expressed only at the transition state for the GPDH-catalyzed reaction. The ratio of rate constants for the phosphite-activated and the unactivated GPDH-catalyzed reduction of GLY by NADH is (4300 M (-2) s (-1))/(8.7 x 10 (-3) M (-1) s (-1)) = 5 x 10 (5) M (-1), which was used to calculate an intrinsic phosphite binding energy of -7.7 kcal/mol for the association of phosphite dianion with the transition state complex for the GPDH-catalyzed reduction of GLY. Phosphite dianion has now been shown to activate bound substrates for enzyme-catalyzed proton transfer, decarboxylation, hydride transfer, and phosphoryl transfer reactions. Structural data provide strong evidence that enzymic activation by the binding of phosphite dianion occurs at a modular active site featuring (1) a binding pocket complementary to the reactive substrate fragment which contains all the active site residues needed to catalyze the reaction of the substrate piece or of the whole substrate and (2) a phosphate/phosphite dianion binding pocket that is completed by the movement of flexible protein loop(s) to surround the nonreacting oxydianion. We propose that loop motion and associated protein conformational changes that accompany the binding of phosphite dianion and/or phosphodianion substrates lead to encapsulation of the substrate and/or its pieces in the protein interior, and to placement of the active site residues in positions where they provide optimal stabilization of the transition state for the catalyzed reaction.
Figures



Similar articles
-
Glycerol 3-Phosphate Dehydrogenase Catalyzed Hydride Transfer: Enzyme Activation by Cofactor Pieces.Biochemistry. 2024 Nov 5;63(21):2878-2891. doi: 10.1021/acs.biochem.4c00324. Epub 2024 Sep 25. Biochemistry. 2024. PMID: 39319842
-
Enzymatic catalysis of proton transfer at carbon: activation of triosephosphate isomerase by phosphite dianion.Biochemistry. 2007 May 15;46(19):5841-54. doi: 10.1021/bi700409b. Epub 2007 Apr 20. Biochemistry. 2007. PMID: 17444661 Free PMC article.
-
Enzyme Architecture: A Startling Role for Asn270 in Glycerol 3-Phosphate Dehydrogenase-Catalyzed Hydride Transfer.Biochemistry. 2016 Mar 15;55(10):1429-32. doi: 10.1021/acs.biochem.6b00116. Epub 2016 Mar 3. Biochemistry. 2016. PMID: 26926520 Free PMC article.
-
Specificity in transition state binding: the Pauling model revisited.Biochemistry. 2013 Mar 26;52(12):2021-35. doi: 10.1021/bi301491r. Epub 2013 Feb 4. Biochemistry. 2013. PMID: 23327224 Free PMC article. Review.
-
Linear Free Energy Relationships for Enzymatic Reactions: Fresh Insight from a Venerable Probe.Acc Chem Res. 2021 May 18;54(10):2532-2542. doi: 10.1021/acs.accounts.1c00147. Epub 2021 May 3. Acc Chem Res. 2021. PMID: 33939414 Free PMC article. Review.
Cited by
-
Rate and Equilibrium Constants for an Enzyme Conformational Change during Catalysis by Orotidine 5'-Monophosphate Decarboxylase.Biochemistry. 2015 Jul 28;54(29):4555-64. doi: 10.1021/acs.biochem.5b00591. Epub 2015 Jul 14. Biochemistry. 2015. PMID: 26135041 Free PMC article.
-
Enzyme Architecture: The Role of a Flexible Loop in Activation of Glycerol-3-phosphate Dehydrogenase for Catalysis of Hydride Transfer.Biochemistry. 2018 Jun 12;57(23):3227-3236. doi: 10.1021/acs.biochem.7b01282. Epub 2018 Feb 5. Biochemistry. 2018. PMID: 29337541 Free PMC article.
-
Dissecting the total transition state stabilization provided by amino acid side chains at orotidine 5'-monophosphate decarboxylase: a two-part substrate approach.Biochemistry. 2008 Jul 29;47(30):7785-7. doi: 10.1021/bi800939k. Epub 2008 Jul 4. Biochemistry. 2008. PMID: 18598058 Free PMC article.
-
Primary Deuterium Kinetic Isotope Effects: A Probe for the Origin of the Rate Acceleration for Hydride Transfer Catalyzed by Glycerol-3-Phosphate Dehydrogenase.Biochemistry. 2018 Jul 24;57(29):4338-4348. doi: 10.1021/acs.biochem.8b00536. Epub 2018 Jul 10. Biochemistry. 2018. PMID: 29927590 Free PMC article.
-
Hydride Transfer Catalyzed by Glycerol Phosphate Dehydrogenase: Recruitment of an Acidic Amino Acid Side Chain to Rescue a Damaged Enzyme.Biochemistry. 2020 Dec 29;59(51):4856-4863. doi: 10.1021/acs.biochem.0c00801. Epub 2020 Dec 11. Biochemistry. 2020. PMID: 33305938 Free PMC article.
References
-
- Pauling L. The nature of forces between large molecules of biological interest. Nature. 1948;161:707–709. - PubMed
-
- Radzicka A, Wolfenden R. A proficient enzyme. Science. 1995;267:90–93. - PubMed
-
- Jencks WP. Binding Energy, Specificity and Enzymic Catalysis: The Circe Effect. Adv. Enzymol. Relat. Areas Mol. Biol. 1975;43:219–410. - PubMed
-
- Jencks WP. Economics of enzyme catalysis. Cold Sp. Har. Sym. Quant. Biol. 1987;52:65–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

