In vitro differentiation of Runx3-/- p53-/- gastric epithelial cells into intestinal type cells
- PMID: 18377419
- PMCID: PMC11160005
- DOI: 10.1111/j.1349-7006.2008.00732.x
In vitro differentiation of Runx3-/- p53-/- gastric epithelial cells into intestinal type cells
Abstract
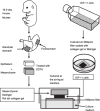
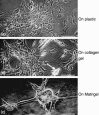
We have reported that a lack of RUNX3 function is causally associated with gastric carcinogenesis. We have also presented evidence that loss of Runx3 may be related to the genesis of intestinal metaplasia because expression of RUNX3 is reduced in some intestinal metaplasias, and some Runx3(-/-)p53(-/-) gastric epithelial cells differentiate into intestinal type cells in vivo. Recently several reports have indicated that blood cells play important roles in the gastric carcinogenesis. In the present study, we therefore examined whether Runx3(-/-)p53(-/-) gastric epithelial cells differentiate autonomously into intestinal type cells, or whether the presence of other cells is necessary for the differentiation in vitro. When Runx3(-/-)p53(-/-) gastric epithelial cells were cultured with collagen gels, they did not exhibit any morphogenesis and differentiated poorly. When cultured with fetal mouse gastric mesenchymes, the cells formed glandular structures and differentiated into surface mucous cells, but differentiation of intestinal type cells was never observed. When cultured with Matrigel, the cells formed glandular structures, and some cells differentiated into intestinal type cells in vitro. Reverse transcription-polymerase chain reaction analysis showed that the cells expressed stomach-specific genes, and their levels decreased gradually during the culture. The cells expressed some intestine-specific genes weakly at the start of culture, and their levels were increased with time in culture. We therefore conclude that Runx3(-/-)p53(-/-) gastric epithelial cells differentiate into intestinal type cells in combination with Matrigel in the absence of other cell types. Extracellular matrix, not blood cells, may play a role in the genesis of intestinal metaplasia.
Figures



Similar articles
-
Runx3-/- gastric epithelial cells differentiate into intestinal type cells.Biochem Biophys Res Commun. 2004 Aug 13;321(1):58-64. doi: 10.1016/j.bbrc.2004.06.099. Biochem Biophys Res Commun. 2004. PMID: 15358215
-
Runx3 controls growth and differentiation of gastric epithelial cells in mammals.Dev Growth Differ. 2006 Jan;48(1):1-13. doi: 10.1111/j.1440-169X.2006.00832.x. Dev Growth Differ. 2006. PMID: 16466388 Review.
-
Runx3 expression in gastrointestinal tract epithelium: resolving the controversy.Oncogene. 2009 Mar 12;28(10):1379-84. doi: 10.1038/onc.2008.496. Epub 2009 Jan 26. Oncogene. 2009. PMID: 19169278
-
Hypermethylation downregulates Runx3 gene expression and its restoration suppresses gastric epithelial cell growth by inducing p27 and caspase3 in human gastric cancer.J Gastroenterol Hepatol. 2010 Apr;25(4):823-31. doi: 10.1111/j.1440-1746.2009.06191.x. J Gastroenterol Hepatol. 2010. PMID: 20492341
-
Helicobacter pylori eradication to prevent gastric cancer: underlying molecular and cellular mechanisms.World J Gastroenterol. 2006 Mar 21;12(11):1671-80. doi: 10.3748/wjg.v12.i11.1671. World J Gastroenterol. 2006. PMID: 16586533 Free PMC article. Review.
Cited by
-
Overexpressed miR-301a promotes cell proliferation and invasion by targeting RUNX3 in gastric cancer.J Gastroenterol. 2013 Sep;48(9):1023-33. doi: 10.1007/s00535-012-0733-6. Epub 2013 Jan 22. J Gastroenterol. 2013. PMID: 23338485
-
Molecular mechanisms of Barrett's esophagus.Dig Dis Sci. 2011 Dec;56(12):3405-20. doi: 10.1007/s10620-011-1885-6. Epub 2011 Oct 8. Dig Dis Sci. 2011. PMID: 21984436 Free PMC article. Review.
-
CD133 is a marker of gland-forming cells in gastric tumors and Sox17 is involved in its regulation.Cancer Sci. 2011 Jul;102(7):1313-21. doi: 10.1111/j.1349-7006.2011.01947.x. Epub 2011 May 9. Cancer Sci. 2011. PMID: 21457403 Free PMC article.
-
Intestinal metaplasia -the effect of Acid on the gastric mucosa and gastric carcinogenesis-.J Toxicol Pathol. 2010 Sep;23(3):115-23. doi: 10.1293/tox.23.115. Epub 2010 Oct 5. J Toxicol Pathol. 2010. PMID: 22272022 Free PMC article.
References
-
- Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999; 83: 18–29. - PubMed
-
- Yuasa Y. Control of gut differentiation and intestinal‐type gastric carcinogenesis. Nature Rev Cancer 2003; 3: 592–600. - PubMed
-
- Stemmermann GN. Intestinal metaplasia of the stomach. Cancer 1994; 74: 556–64. - PubMed
-
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process. Cancer Res 1992; 52: 6735–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

