Rearranged NF-kappa B2 gene in an adult T-cell leukemia cell line
- PMID: 18377428
- PMCID: PMC11159331
- DOI: 10.1111/j.1349-7006.2008.00750.x
Rearranged NF-kappa B2 gene in an adult T-cell leukemia cell line
Abstract
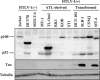
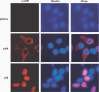
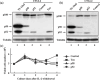
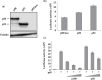
Adult T-cell leukemia (ATL) is an aggressive type of leukemia, originating from T-cells infected with human T-cell leukemia virus type 1. Accumulating evidence suggests the aberrant activation of NF-kappaB to be a causative factor mediating the abnormal proliferation of leukemic cells, thus resulting in the development of ATL. A rearranged NF-kappa B2/p100 gene was isolated from an ATL-derived cell line, which was generated by a chromosomal translocation. The isolated NF-kappa B2 mutant is fused with the with no (lysine) deficient protein kinase 1 gene, coding for a 58 kDa protein that retains the DNA binding Rel homology domain, but it lacks the entire ankyrin repeat inhibitory domain, thus suggesting its constitutive activation. This rearranged NF-kappa B2 gene product (p58) was localized in the nucleus, and formed a complex with NF-kappaB p65 or RelB. Moreover, a T-cell line expressing p58 increased the amount of an NF-kappa B2-inducible gene, NF-kappa B2/p100 by itself. These results suggest that such NF-kappa B2 gene rearrangement may therefore be a factor in the constitutive activation of NF-kappaB in ATL, and thereby playing a role in the ATL pathogenesis.
Figures



Similar articles
-
Notch3 and pre-TCR interaction unveils distinct NF-kappaB pathways in T-cell development and leukemia.EMBO J. 2006 Mar 8;25(5):1000-8. doi: 10.1038/sj.emboj.7600996. Epub 2006 Feb 23. EMBO J. 2006. PMID: 16498412 Free PMC article.
-
Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells.Blood. 1999 Apr 1;93(7):2360-8. Blood. 1999. PMID: 10090947
-
A roadmap of constitutive NF-κB activity in Hodgkin lymphoma: Dominant roles of p50 and p52 revealed by genome-wide analyses.Genome Med. 2016 Mar 17;8(1):28. doi: 10.1186/s13073-016-0280-5. Genome Med. 2016. PMID: 26988706 Free PMC article.
-
Immune Differentiation Regulator p100 Tunes NF-κB Responses to TNF.Front Immunol. 2019 May 7;10:997. doi: 10.3389/fimmu.2019.00997. eCollection 2019. Front Immunol. 2019. PMID: 31134075 Free PMC article.
-
NF-κB-Induced R-Loops and Genomic Instability in HTLV-1-Infected and Adult T-Cell Leukemia Cells.Viruses. 2022 Apr 23;14(5):877. doi: 10.3390/v14050877. Viruses. 2022. PMID: 35632619 Free PMC article. Review.
Cited by
-
Human T-cell lymphotropic virus: a model of NF-κB-associated tumorigenesis.Viruses. 2011 Jun;3(6):714-49. doi: 10.3390/v3060714. Viruses. 2011. PMID: 21743832 Free PMC article. Review.
-
NF-κB and cancer: a paradigm of Yin-Yang.Am J Cancer Res. 2011;1(2):192-221. Epub 2010 Dec 6. Am J Cancer Res. 2011. PMID: 21969033 Free PMC article.
-
The noncanonical NFκB pathway: Regulatory mechanisms in health and disease.WIREs Mech Dis. 2024 Nov-Dec;16(6):e1646. doi: 10.1002/wsbm.1646. Epub 2024 Apr 18. WIREs Mech Dis. 2024. PMID: 38634218 Review.
-
Identification of a novel motif responsible for the distinctive transforming activity of human T-cell leukemia virus (HTLV) type 1 Tax1 protein from HTLV-2 Tax2.Retrovirology. 2009 Sep 17;6:83. doi: 10.1186/1742-4690-6-83. Retrovirology. 2009. PMID: 19761585 Free PMC article.
-
NF-κB Activation in Lymphoid Malignancies: Genetics, Signaling, and Targeted Therapy.Biomedicines. 2018 Mar 26;6(2):38. doi: 10.3390/biomedicines6020038. Biomedicines. 2018. PMID: 29587428 Free PMC article. Review.
References
-
- Uchiyama T, Yodoi J, Sagawa K et al . Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood 1977; 50: 481–92. - PubMed
-
- Matsuoka M, Jeang KT. Human T‐cell leukaemia virus type 1 (HTLV‐1) infectivity and cellular transformation. Nat Rev Cancer 2007; 7: 270–80. - PubMed
-
- Giam CZ, Jeang KT. HTLV‐1 Tax and adult T‐cell leukemia. Front Biosci 2007; 12: 1496–507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

