Brain asymmetry is encoded at the level of axon terminal morphology
- PMID: 18377638
- PMCID: PMC2292717
- DOI: 10.1186/1749-8104-3-9
Brain asymmetry is encoded at the level of axon terminal morphology
Abstract
Background: Functional lateralization is a conserved feature of the central nervous system (CNS). However, underlying left-right asymmetries within neural circuitry and the mechanisms by which they develop are poorly described.
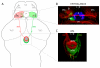
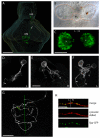
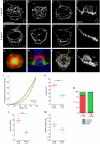
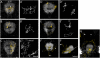
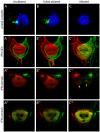
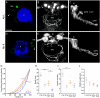
Results: In this study, we use focal electroporation to examine the morphology and connectivity of individual neurons of the lateralized habenular nuclei. Habenular projection neurons on both sides of the brain share a stereotypical unipolar morphology and elaborate remarkable spiraling terminal arbors in their target interpeduncular nucleus, a morphology unlike that of any other class of neuron described to date. There are two quite distinct sub-types of axon arbor that differ both in branching morphology and in their localization within the target nucleus. Critically, both arbor morphologies are elaborated by both left and right-sided neurons, but at greatly differing frequencies. We show that these differences in cell type composition account for the gross connectional asymmetry displayed by the left and right habenulae. Analysis of the morphology and projections of individual post-synaptic neurons suggests that the target nucleus has the capacity to either integrate left and right inputs or to handle them independently, potentially relaying information from the left and right habenulae within distinct downstream pathways, thus preserving left-right coding. Furthermore, we find that signaling from the unilateral, left-sided parapineal nucleus is necessary for both left and right axons to develop arbors with appropriate morphology and targeting. However, following parapineal ablation, left and right habenular neurons continue to elaborate arbors with distinct, lateralized morphologies.
Conclusion: By taking the analysis of asymmetric neural circuitry to the level of single cells, we have resolved left-right differences in circuit microarchitecture and show that lateralization can be recognized at the level of the morphology and connectivity of single projection neuron axons. Crucially, the same circuitry components are specified on both sides of the brain, but differences in the ratios of different neuronal sub-types results in a lateralized neural architecture and gross connectional asymmetry. Although signaling from the parapineal is essential for the development of normal lateralization, additional factors clearly act during development to confer left-right identity upon neurons in this highly conserved circuit.
Figures
References
-
- Rogers LJ, Andrew RJ. Comparitive Vertebrate Lateralization. Cambridge, Cambridge University Press; 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

