Renal safety of zoledronic acid with thalidomide in patients with myeloma: a pharmacokinetic and safety sub-study
- PMID: 18377658
- PMCID: PMC2330021
- DOI: 10.1186/1472-6904-8-2
Renal safety of zoledronic acid with thalidomide in patients with myeloma: a pharmacokinetic and safety sub-study
Abstract
Background: Cases of impaired renal function have been reported in patients who had been treated with both zoledronic acid and thalidomide for myeloma. Hence, we conducted a safety study of zoledronic acid and thalidomide in myeloma patients participating in a trial of maintenance therapy.
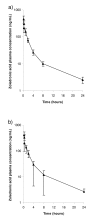
Methods: Twenty-four patients who were enrolled in a large randomized trial of thalidomide vs no thalidomide maintenance therapy for myeloma, in which all patients also received zoledronic acid, were recruited to a pharmacokinetic and renal safety sub-study, and followed for up to 16 months.
Results: No significant differences by Wilcoxon rank-sum statistic were found in zoledronic acid pharmacokinetics or renal safety for up to 16 months in patients randomized to thalidomide or not.
Conclusion: In myeloma patients receiving maintenance therapy, the combination of zoledronic acid and thalidomide appears to confer no additional renal safety risks over zoledronic acid alone.
Figures

References
-
- Legay F, Gauron S, Deckert F, Gosset G, Pfaar U, Ravera C, Wiegand H, Schran H. Development and validation of a highly sensitive RIA for zoledronic acid, a new potent heterocyclic bisphosphonate, in human serum, plasma and urine. J Pharm Biomed Anal. 2002;30:897–911. doi: 10.1016/S0731-7085(02)00218-2. - DOI - PubMed
-
- Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, Schran H, Seaman J, Skerjanec A. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42:1228–36. doi: 10.1177/009127002762491316. - DOI - PubMed
-
- Berenson JR, Vescio RA, Rosen LS, VonTeichert JM, Woo M, Swift R, Savage A, Givant E, Hupkes M, Harvey H, Lipton A. A Phase I dose-ranging trial of monthly infusions of zoledronic acid for the treatment of osteolytic bone metastases. Clin Cancer Res. 2001;7:478–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

