Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF Receptor-2 and attenuates endothelial DNA synthesis, but not migration*
- PMID: 18377662
- PMCID: PMC2292718
- DOI: 10.1186/1750-2187-3-8
Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF Receptor-2 and attenuates endothelial DNA synthesis, but not migration*
Abstract
Background: Vascular endothelial growth factor receptor-2 (VEGFR-2, KDR), a receptor tyrosine kinase, regulates mitogenic, chemotactic, hyperpermeability, and survival signals in vascular endothelial cells in response to its ligand vascular permeability factor/ vascular endothelial growth factor (VPF/VEGF). SHP-1 is a protein tyrosine phosphatase known to negatively regulate signaling from receptors such as EGF receptor, IL3 receptor, erythropoietin receptor and also KDR. However, the mechanism by which SHP-1 executes KDR dephosphorylation, the targeted tyrosine residue(s) of KDR and also overall downstream signaling or phenotypic change(s) caused, is not defined.
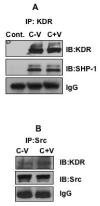
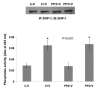
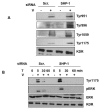
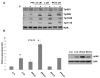
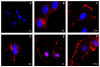
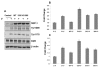
Results: Here, we have demonstrated that KDR and SHP-1 are constitutively associated and upon VEGF treatment, the phosphatase activity of SHP-1 is stimulated in a c-Src kinase dependent manner. Knockdown of SHP-1 by siRNA or inhibition of c-Src by an inhibitor, results in augmented DNA synthesis perhaps due to increased phosphorylation of at least three tyrosine residues of KDR 996, 1059 and 1175. On the other hand, neither tyrosine residue 951 of KDR nor VEGF-mediated migration is affected by modulation of SHP-1 function.
Conclusion: Taken together our results define the tyrosine residues of KDR that are regulated by SHP-1 and also elucidates a novel feed back loop where SHP-1 is activated upon VEGF treatment through c-Src and controls KDR induced DNA synthesis, eventually leading to controlled angiogenesis.
Figures


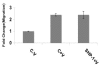
References
-
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. - PubMed
-
- Wizigmann-Voos S, Breier G, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor and its receptors in von Hippel-Lindau disease-associated and sporadic hemangioblastomas. Cancer Res. 1995;55:1358–1364. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

