Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells
- PMID: 18377871
- PMCID: PMC2424318
- DOI: 10.1016/j.bcp.2008.02.023
Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells
Abstract
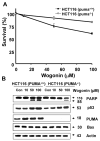
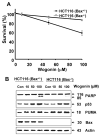
We observed that treatment of prostate cancer cells for 24 h with wogonin, a naturally occurring monoflavonoid, induced cell death in a dose- and time-dependent manner. Exposure of wogonin to LNCaP cells was associated with increased intracellular levels of p21(Cip-1), p27(Kip-1), p53, and PUMA, oligomerization of Bax, release of cytochrome c from the mitochondria, and activation of caspases. We also confirmed the role of p53 by noting that knock-in in p53 expression by transfecting p53 DNA increased wogonin-induced apoptosis in p53-null PC-3 cells. To study the mechanism of PUMA up-regulation, we determined the activities of PUMA promoter in the wogonin treated and untreated cells. Increase of the intracellular levels of PUMA protein was due to increase in transcriptional activity. Data from chromatin immunoprecipitation (ChIP) analyses revealed that wogonin activated the transcription factor p53 binding activity to the PUMA promoter region. We observed that the up-regulation of PUMA mediated wogonin cytotoxicity. Further characterization of the transcriptional response to wogonin in HCT116 human colon cancer cells demonstrated that PUMA induction was p53-dependent; deficiency in either p53 or PUMA significantly protected HCT116 cells against wogonin-induced apoptosis. Also, wogonin promoted mitochondrial translocation and multimerization of Bax. Interestingly, wogonin (100 microM) treatment did not affect the viability of normal human prostate epithelial cells (PrEC). Taken together, these results indicate that p53-dependent transcriptional induction of PUMA and oligomerization of Bax play important roles in the sensitivity of cancer cells to apoptosis induced by caspase activation through wogonin.
Figures







Similar articles
-
Reactive oxygen species up-regulate p53 and Puma; a possible mechanism for apoptosis during combined treatment with TRAIL and wogonin.Br J Pharmacol. 2009 Aug;157(7):1189-202. doi: 10.1111/j.1476-5381.2009.00245.x. Epub 2009 May 11. Br J Pharmacol. 2009. PMID: 19438509 Free PMC article.
-
Systematic genetic dissection of p14ARF-mediated mitochondrial cell death signaling reveals a key role for p21CDKN1 and the BH3-only protein Puma/bbc3.J Mol Med (Berl). 2010 Jun;88(6):609-22. doi: 10.1007/s00109-010-0606-5. Epub 2010 Apr 25. J Mol Med (Berl). 2010. PMID: 20419447
-
p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation.J Biol Chem. 2004 Feb 27;279(9):8076-83. doi: 10.1074/jbc.M307469200. Epub 2003 Nov 21. J Biol Chem. 2004. PMID: 14634023
-
A coordinated action of Bax, PUMA, and p53 promotes MG132-induced mitochondria activation and apoptosis in colon cancer cells.Mol Cancer Ther. 2007 Mar;6(3):1062-9. doi: 10.1158/1535-7163.MCT-06-0541. Mol Cancer Ther. 2007. PMID: 17363499
-
PUMA, a potent killer with or without p53.Oncogene. 2008 Dec;27 Suppl 1(Suppl 1):S71-83. doi: 10.1038/onc.2009.45. Oncogene. 2008. PMID: 19641508 Free PMC article. Review.
Cited by
-
Molecular mechanism and therapeutic potential of HDAC9 in intervertebral disc degeneration.Cell Mol Biol Lett. 2023 Dec 13;28(1):104. doi: 10.1186/s11658-023-00517-x. Cell Mol Biol Lett. 2023. PMID: 38093179 Free PMC article.
-
A Novel Prostate-Specific Membrane-Antigen (PSMA) Targeted Micelle-Encapsulating Wogonin Inhibits Prostate Cancer Cell Proliferation via Inducing Intrinsic Apoptotic Pathway.Int J Mol Sci. 2016 May 17;17(5):676. doi: 10.3390/ijms17050676. Int J Mol Sci. 2016. PMID: 27196894 Free PMC article.
-
Wogonin induces reactive oxygen species production and cell apoptosis in human glioma cancer cells.Int J Mol Sci. 2012;13(8):9877-9892. doi: 10.3390/ijms13089877. Epub 2012 Aug 8. Int J Mol Sci. 2012. PMID: 22949836 Free PMC article.
-
Wogonin Increases Cisplatin Sensitivity in Ovarian Cancer Cells Through Inhibition of the Phosphatidylinositol 3-Kinase (PI3K)/Akt Pathway.Med Sci Monit. 2019 Aug 12;25:6007-6014. doi: 10.12659/MSM.913829. Med Sci Monit. 2019. PMID: 31402794 Free PMC article.
-
Methylwogonin exerts anticancer effects in A375 human malignant melanoma cells through apoptosis induction, DNA damage, cell invasion inhibition and downregulation of the mTOR/PI3K/Akt signalling pathway.Arch Med Sci. 2019 Jul;15(4):1056-1064. doi: 10.5114/aoms.2018.73711. Epub 2018 Apr 16. Arch Med Sci. 2019. PMID: 31360200 Free PMC article.
References
-
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics. CA - Cancer J Clin. 2000;50:7–33. - PubMed
-
- Carter HB, Coffey DS. The prostate: an increasing medical problem. Prostate. 1990;16:39–48. - PubMed
-
- Garnick MB. Hormonal therapy in the management of prostate cancer: from Higgins to the present. Urology. 1997;49:5–15. - PubMed
-
- Albertsen PC, Fryback DG, Storere BE. Long-term survival among men with conservatively treated localized prostate cancer. J Am Med Assoc. 1995;274:626–31. - PubMed
-
- Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

