Hyperpolarization-activated current (I(h)) contributes to excitability of primary sensory neurons in rats
- PMID: 18377879
- PMCID: PMC2745653
- DOI: 10.1016/j.brainres.2008.02.066
Hyperpolarization-activated current (I(h)) contributes to excitability of primary sensory neurons in rats
Abstract
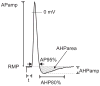
In various excitable tissues, the hyperpolarization-activated, cyclic nucleotide-gated current (I(h)) contributes to burst firing by depolarizing the membrane after a period of hyperpolarization. Alternatively, conductance through open channels I(h) channels of the resting membrane may impede excitability. Since primary sensory neurons of the dorsal root ganglion show both loss of I(h) and elevated excitability after peripheral axonal injury, we examined the contribution of I(h) to excitability of these neurons. We used a sharp electrode intracellular technique to record from neurons in nondissociated ganglia to avoid potential artefacts due to tissue dissociation and cytosolic dialysis. Neurons were categorized by conduction velocity. I(h) induced by hyperpolarizing voltage steps was completely blocked by ZD7288 (approximately 10 microM), which concurrently eliminated the depolarizing sag of transmembrane potential during hyperpolarizing current injection. I(h) was most prominent in rapidly conducting Aalpha/beta neurons, in which ZD7288 produced resting membrane hyperpolarization, slowed conduction velocity, prolonged action potential (AP) duration, and elevated input resistance. The rheobase current necessary to trigger an AP was elevated and repetitive firing was inhibited by ZD7288, indicating an excitatory influence of I(h). Less I(h) was evident in more slowly conducting Adelta neurons, resulting in diminished effects of ZD7288 on AP parameters. Repetitive firing in these neurons was also inhibited by ZD7288, and the peak frequency of AP transmission during tetanic bursts was diminished by ZD7288. Slowly conducting C-type neurons showed minimal I(h), and no effect of ZD7288 on excitability was seen. After spinal nerve ligation, axotomized neurons had less I(h) compared to control neurons and showed minimal effects of ZD7288 application. We conclude that I(h) supports sensory neuron excitability, and loss of I(h) is not a factor contributing to increased neuronal excitability after peripheral axonal injury.
Figures


Similar articles
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
Functional impact of the hyperpolarization-activated current on the excitability of myelinated A-type vagal afferent neurons in the rat.Clin Exp Pharmacol Physiol. 2010 Aug;37(8):852-61. doi: 10.1111/j.1440-1681.2010.05396.x. Epub 2010 Apr 26. Clin Exp Pharmacol Physiol. 2010. PMID: 20456426 Free PMC article.
-
Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats.Anesthesiology. 2005 Aug;103(2):360-76. doi: 10.1097/00000542-200508000-00020. Anesthesiology. 2005. PMID: 16052119
-
Hyperpolarization-activated, cation-nonselective, cyclic nucleotide-modulated channel blockade alleviates mechanical allodynia and suppresses ectopic discharge in spinal nerve ligated rats.J Pain. 2005 Jul;6(7):417-24. doi: 10.1016/j.jpain.2005.02.002. J Pain. 2005. PMID: 15993819
-
Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain.Croat Med J. 2007 Feb;48(1):9-21. Croat Med J. 2007. PMID: 17309135 Free PMC article. Review.
Cited by
-
Nerve injury induces a new profile of tactile and mechanical nociceptor input from undamaged peripheral afferents.J Neurophysiol. 2015 Jan 1;113(1):100-9. doi: 10.1152/jn.00506.2014. Epub 2014 Oct 1. J Neurophysiol. 2015. PMID: 25274350 Free PMC article.
-
Subtype-specific reduction of voltage-gated calcium current in medium-sized dorsal root ganglion neurons after painful peripheral nerve injury.Neuroscience. 2011 Apr 14;179:244-55. doi: 10.1016/j.neuroscience.2011.01.049. Epub 2011 Jan 28. Neuroscience. 2011. PMID: 21277351 Free PMC article.
-
Partial nerve injury induces electrophysiological changes in conducting (uninjured) nociceptive and nonnociceptive DRG neurons: Possible relationships to aspects of peripheral neuropathic pain and paresthesias.Pain. 2012 Sep;153(9):1824-1836. doi: 10.1016/j.pain.2012.04.019. Epub 2012 Jun 20. Pain. 2012. PMID: 22721911 Free PMC article.
-
Functional subgroups of rat and human sensory neurons: a systematic review of electrophysiological properties.Pflugers Arch. 2022 Apr;474(4):367-385. doi: 10.1007/s00424-021-02656-6. Epub 2022 Jan 15. Pflugers Arch. 2022. PMID: 35031856 Free PMC article.
-
Novel role of KT5720 on regulating hyperpolarization-activated cyclic nucleotide-gated channel activity and dorsal root ganglion neuron excitability.DNA Cell Biol. 2013 Jun;32(6):320-8. doi: 10.1089/dna.2013.2021. DNA Cell Biol. 2013. PMID: 23713946 Free PMC article.
References
-
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. Journal of Neurophysiology. 2001;85:644–58. - PubMed
-
- Baccei ML, Kocsis JD. Voltage-gated calcium currents in axotomized adult rat cutaneous afferent neurons. Journal of Neurophysiology. 2000;83:2227–38. - PubMed
-
- Birch BD, Kocsis JD, Di Gregorio F, Bhisitkul RB, Waxman SG. A voltage- and time-dependent rectification in rat dorsal spinal root axons. Journal of Neurophysiology. 1991;66:719–28. - PubMed
-
- Blatt MR, Slayman CL. KCl leakage from microelectrodes and its impact on the membrane parameters of a nonexcitable cell. Journal of Membrane Biology. 1983;72:223–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

