Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway
- PMID: 18378669
- PMCID: PMC2386912
- DOI: 10.1074/jbc.M801070200
Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway
Abstract
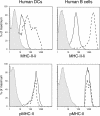
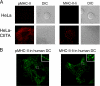
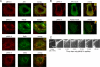
Major histocompatibility complex (MHC) class II molecules (MHC-II) function by binding antigenic peptides and displaying these peptides on the surface of antigen presenting cells (APCs) for recognition by peptide-MHC-II (pMHC-II)-specific CD4 T cells. It is known that cell surface MHC-II can internalize, exchange antigenic peptides in endosomes, and rapidly recycle back to the plasma membrane; however, the molecular machinery and trafficking pathways utilized by internalizing/recycling MHC-II have not been identified. We now demonstrate that unlike newly synthesized invariant chain-associated MHC-II, mature cell surface pMHC-II complexes internalize following clathrin-, AP-2-, and dynamin-independent endocytosis pathways. Immunofluorescence microscopy of MHC-II expressing HeLa-CIITA cells, human B cells, and human DCs revealed that pMHC enters Arf6(+)Rab35(+)EHD1(+) tubular endosomes following endocytosis. These data contrast the internalization pathways followed by newly synthesized and peptide-loaded MHC-II molecules and demonstrates that cell surface pMHC-II internalize and rapidly recycle from early endocytic compartments in tubular endosomes.
Figures

Similar articles
-
Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments.Proc Natl Acad Sci U S A. 2005 May 31;102(22):7910-5. doi: 10.1073/pnas.0502206102. Epub 2005 May 23. Proc Natl Acad Sci U S A. 2005. PMID: 15911768 Free PMC article.
-
CD1a and MHC class I follow a similar endocytic recycling pathway.Traffic. 2008 Sep;9(9):1446-57. doi: 10.1111/j.1600-0854.2008.00781.x. Epub 2008 Jun 28. Traffic. 2008. PMID: 18564371 Free PMC article.
-
The adaptor complex AP-2 regulates post-endocytic trafficking through the non-clathrin Arf6-dependent endocytic pathway.J Cell Sci. 2008 Dec 15;121(Pt 24):4008-17. doi: 10.1242/jcs.033522. Epub 2008 Nov 25. J Cell Sci. 2008. PMID: 19033387
-
Trafficking of MHC class II molecules in the late secretory pathway.Curr Opin Immunol. 2002 Feb;14(1):30-5. doi: 10.1016/s0952-7915(01)00295-3. Curr Opin Immunol. 2002. PMID: 11790530 Review.
-
Antigen presentation by MHC class II molecules.Immunol Cell Biol. 1997 Feb;75(1):69-81. doi: 10.1038/icb.1997.11. Immunol Cell Biol. 1997. PMID: 9046437 Review.
Cited by
-
Virus Control of Trafficking from Sorting Endosomes.mBio. 2018 Jul 24;9(4):e00683-18. doi: 10.1128/mBio.00683-18. mBio. 2018. PMID: 30042195 Free PMC article.
-
Hsp90-peptide complexes stimulate antigen presentation through the class II pathway after binding scavenger receptor SREC-I.Immunobiology. 2014 Dec;219(12):924-31. doi: 10.1016/j.imbio.2014.08.001. Epub 2014 Aug 10. Immunobiology. 2014. PMID: 25155057 Free PMC article.
-
Dendritic cells utilize the evolutionarily conserved WASH and retromer complexes to promote MHCII recycling and helper T cell priming.PLoS One. 2014 Jun 2;9(6):e98606. doi: 10.1371/journal.pone.0098606. eCollection 2014. PLoS One. 2014. PMID: 24886983 Free PMC article.
-
Pathways and mechanisms of endocytic recycling.Nat Rev Mol Cell Biol. 2009 Sep;10(9):597-608. doi: 10.1038/nrm2755. Nat Rev Mol Cell Biol. 2009. PMID: 19696797 Free PMC article. Review.
-
Quantitating MHC class II trafficking in primary dendritic cells using imaging flow cytometry.J Immunol Methods. 2015 Aug;423:18-28. doi: 10.1016/j.jim.2015.04.023. Epub 2015 May 9. J Immunol Methods. 2015. PMID: 25967952 Free PMC article.
References
-
- Trombetta, E. S., and Mellman, I. (2005) Annu. Rev. Immunol. 23 975-1028 - PubMed
-
- von Delwig, A., Ramachandra, L., Harding, C. V., and Robinson, J. H. (2003) Eur. J. Immunol. 33 2353-2360 - PubMed
-
- von Delwig, A., Musson, J. A., Shim, H. K., Lee, J. J., Walker, N., Harding, C. V., Williamson, E. D., and Robinson, J. H. (2005) Scand. J. Immunol. 62 243-250 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

