Potent synergistic anti-human immunodeficiency virus (HIV) effects using combinations of the CCR5 inhibitor aplaviroc with other anti-HIV drugs
- PMID: 18378711
- PMCID: PMC2415794
- DOI: 10.1128/AAC.01299-07
Potent synergistic anti-human immunodeficiency virus (HIV) effects using combinations of the CCR5 inhibitor aplaviroc with other anti-HIV drugs
Abstract
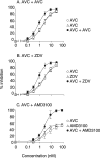
Aplaviroc (AVC), an experimental CCR5 inhibitor, potently blocks in vitro the infection of R5-tropic human immunodeficiency virus type 1 (R5-HIV-1) at subnanomolar 50% inhibitory concentrations. Although maraviroc is presently clinically available, further studies are required to determine the role of CCR5 inhibitors in combinations with other drugs. Here we determined anti-HIV-1 activity using combinations of AVC with various anti-HIV-1 agents, including four U.S. Food and Drug Administration-approved drugs, two CCR5 inhibitors (TAK779 and SCH-C) and two CXCR4 inhibitors (AMD3100 and TE14011). Combination effects were defined as synergistic or antagonistic when the activity of drug A combined with B was statistically greater or less, respectively, than the additive effects of drugs A and A combined and drugs B and B combined by using the Combo method, described in this paper, which provides (i) a flexible choice of interaction models and (ii) the use of nonparametric statistical methods. Synergistic effects against R5-HIV-1(Ba-L) and a 50:50 mixture of R5-HIV-1(Ba-L) and X4-HIV-1(ERS104pre) (HIV-1(Ba-L/104pre)) were seen when AVC was combined with zidovudine, nevirapine, indinavir, or enfuvirtide. Mild synergism and additivity were observed when AVC was combined with TAK779 and SCH-C, respectively. We also observed more potent synergism against HIV-1(Ba-L/104pre) when AVC was combined with AMD3100 or TE14011. The data demonstrate a tendency toward greater synergism with AVC plus either of the two CXCR4 inhibitors compared to the synergism obtained with combinations of AVC and other drugs, suggesting that the development of effective CXCR4 inhibitors may be important for increasing the efficacies of CCR5 inhibitors.
Figures




Similar articles
-
Distinct efficacy of HIV-1 entry inhibitors to prevent cell-to-cell transfer of R5 and X4 viruses across a human placental trophoblast barrier in a reconstitution model in vitro.Retrovirology. 2008 Mar 31;5:31. doi: 10.1186/1742-4690-5-31. Retrovirology. 2008. PMID: 18377645 Free PMC article.
-
Characterization of a human immunodeficiency virus type 1 V3 deletion mutation that confers resistance to CCR5 inhibitors and the ability to use aplaviroc-bound receptor.J Virol. 2009 Apr;83(8):3798-809. doi: 10.1128/JVI.01751-08. Epub 2009 Feb 4. J Virol. 2009. PMID: 19193800 Free PMC article.
-
Inhibition of dual/mixed tropic HIV-1 isolates by CCR5-inhibitors in primary lymphocytes and macrophages.PLoS One. 2013 Jul 9;8(7):e68076. doi: 10.1371/journal.pone.0068076. Print 2013. PLoS One. 2013. PMID: 23874501 Free PMC article.
-
HIV co-receptors as targets for antiviral therapy.Curr Top Med Chem. 2004;4(9):883-93. doi: 10.2174/1568026043388501. Curr Top Med Chem. 2004. PMID: 15134547 Review.
-
[Viral entry as therapeutic target. Current situation of entry inhibitors].Enferm Infecc Microbiol Clin. 2008 Oct;26 Suppl 11:5-11. doi: 10.1016/s0213-005x(08)76557-1. Enferm Infecc Microbiol Clin. 2008. PMID: 19133215 Review. Spanish.
Cited by
-
Closing the door to human immunodeficiency virus.Protein Cell. 2013 Feb;4(2):86-102. doi: 10.1007/s13238-012-2111-9. Epub 2013 Mar 12. Protein Cell. 2013. PMID: 23479426 Free PMC article. Review.
-
Discovery of Rift Valley fever virus natural pan-inhibitors by targeting its multiple key proteins through computational approaches.Sci Rep. 2022 Jun 3;12(1):9260. doi: 10.1038/s41598-022-13267-1. Sci Rep. 2022. PMID: 35662263 Free PMC article.
-
Computational screening of medicinal plant phytochemicals to discover potent pan-serotype inhibitors against dengue virus.Sci Rep. 2019 Feb 5;9(1):1433. doi: 10.1038/s41598-018-38450-1. Sci Rep. 2019. PMID: 30723263 Free PMC article.
-
Insights into the mechanism of inhibition of CXCR4: identification of Piperidinylethanamine analogs as anti-HIV-1 inhibitors.Antimicrob Agents Chemother. 2015 Apr;59(4):1895-904. doi: 10.1128/AAC.04654-14. Epub 2015 Jan 12. Antimicrob Agents Chemother. 2015. PMID: 25583709 Free PMC article.
-
Env-glycoprotein heterogeneity as a source of apparent synergy and enhanced cooperativity in inhibition of HIV-1 infection by neutralizing antibodies and entry inhibitors.Virology. 2012 Jan 5;422(1):22-36. doi: 10.1016/j.virol.2011.09.019. Epub 2011 Oct 22. Virology. 2012. PMID: 22018634 Free PMC article.
References
-
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
-
- Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. - PMC - PubMed
-
- Chou, T. C., and P. Talalay. 1981. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur. J. Biochem. 115:207-216. - PubMed
-
- Chou, T. C., and P. Talalay. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27-55. - PubMed
-
- Daar, E. S., K. L. Kesler, C. J. Petropoulos, W. Huang, M. Bates, A. E. Lail, E. P. Coakley, E. D. Gomperts, and S. M. Donfield. 2007. Baseline HIV type 1 coreceptor tropism predicts disease progression. Clin. Infect. Dis. 45:643-649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

