Cerebrospinal fluid compartmental pharmacokinetics of amikacin in neonates
- PMID: 18378715
- PMCID: PMC2415815
- DOI: 10.1128/AAC.01099-07
Cerebrospinal fluid compartmental pharmacokinetics of amikacin in neonates
Abstract
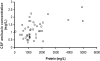
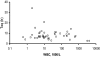
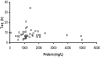
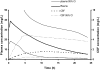
To describe and investigate the covariate effects of cerebrospinal fluid (CSF) amikacin pharmacokinetics in neonates, CSF samples were prospectively collected from neonates in whom amikacin had been initiated before a diagnostic lumbar puncture was performed. CSF analysis (amikacin concentration, white blood count [WBC], glucose content, and protein concentration) and amikacin therapeutic drug monitoring results (peak and trough concentrations) in serum were recorded. Correlations (Spearman rank) between the CSF amikacin concentration and the CSF WBC and glucose and protein concentration were investigated. There were 44 CSF amikacin concentrations and 83 serum samples available from 43 neonates (mean postmenstrual age, 36 weeks [range, 26 to 41 weeks]; mean weight, 2.43 kg [range, 0.87 to 3.86 kg]). The median time interval between initiation of amikacin administration and CSF sampling was 25 h (range, 2.5 to 93.7 h). The median amikacin concentration in the CSF was 1.08 mg/liter (range, 0.34 to 2.65 mg/liter), and the mean trough and peak amikacin concentrations in serum were 3.8 +/- 2.5 mg/liter and 35.7 +/- 5.9 mg/liter, respectively. A correlation between CSF amikacin and CSF protein contents (P < 0.01, r = 0.41, 95% confidence interval = 0.13 to 0.63) but not between CSF WBC and CSF glucose was documented. A two-compartment (central and CSF) linear disposition model was used to estimate population pharmacokinetics. The half time for equilibration (T(eq)) between serum and CSF compartments was used as a measure of blood-brain barrier permeability. The T(eq) was 7.58 h (coefficient of variation [CV] = 49.1%) with a partition coefficient of 0.103 (CV = 26.4%). There was no relationship between the T(eq) and CSF WBC, CSF glucose content, or CSF protein content.
Figures
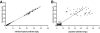
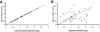

Similar articles
-
Cerebrospinal fluid penetration of amikacin in children with community-acquired bacterial meningitis.Antimicrob Agents Chemother. 1995 Jan;39(1):253-5. doi: 10.1128/AAC.39.1.253. Antimicrob Agents Chemother. 1995. PMID: 7695318 Free PMC article.
-
Effect of delay in analysis on neonatal cerebrospinal fluid parameters.Arch Dis Child Fetal Neonatal Ed. 2010 Jan;95(1):F25-9. doi: 10.1136/adc.2008.150292. Epub 2009 Aug 10. Arch Dis Child Fetal Neonatal Ed. 2010. PMID: 19671531
-
Prospective Evaluation of a Model-Based Dosing Regimen for Amikacin in Preterm and Term Neonates in Clinical Practice.Antimicrob Agents Chemother. 2015 Oct;59(10):6344-51. doi: 10.1128/AAC.01157-15. Epub 2015 Jul 27. Antimicrob Agents Chemother. 2015. PMID: 26248375 Free PMC article.
-
Lumbar puncture in the neonate: challenges in decision making and interpretation.Semin Perinatol. 2012 Dec;36(6):445-53. doi: 10.1053/j.semperi.2012.06.007. Semin Perinatol. 2012. PMID: 23177804 Review.
-
Antibiotic pharmacodynamics in cerebrospinal fluid.Clin Infect Dis. 1998 Nov;27(5):1117-27, quiz 1128-9. doi: 10.1086/515003. Clin Infect Dis. 1998. PMID: 9827256 Review.
Cited by
-
Assessment of flomoxef combined with amikacin in a hollow-fibre infection model for the treatment of neonatal sepsis in low- and middle-income healthcare settings.J Antimicrob Chemother. 2022 Nov 28;77(12):3349-3357. doi: 10.1093/jac/dkac323. J Antimicrob Chemother. 2022. PMID: 36177766 Free PMC article.
-
Potential Antibiotics for the Treatment of Neonatal Sepsis Caused by Multidrug-Resistant Bacteria.Paediatr Drugs. 2021 Sep;23(5):465-484. doi: 10.1007/s40272-021-00465-z. Epub 2021 Aug 26. Paediatr Drugs. 2021. PMID: 34435316 Free PMC article. Review.
-
Pharmacokinetic studies in infants using minimal-risk study designs.Curr Clin Pharmacol. 2014;9(4):350-8. doi: 10.2174/1574884709666140520153308. Curr Clin Pharmacol. 2014. PMID: 24844642 Free PMC article. Review.
-
Tissue Penetration of Antimicrobials in Intensive Care Unit Patients: A Systematic Review-Part II.Antibiotics (Basel). 2022 Sep 3;11(9):1193. doi: 10.3390/antibiotics11091193. Antibiotics (Basel). 2022. PMID: 36139972 Free PMC article. Review.
-
Quantification of amikacin in bronchial epithelial lining fluid in neonates.Antimicrob Agents Chemother. 2011 Sep;55(9):3990-3. doi: 10.1128/AAC.00277-11. Epub 2011 Jun 27. Antimicrob Agents Chemother. 2011. PMID: 21709076 Free PMC article.
References
-
- Allegaert, K., V. Cossey, J. P. Langhendries, G. Naulaers, C. Vanhole, H. Devlieger, and B. Van Overmeire. 2004. Effects of co-administration of ibuprofen-lysine on the pharmacokinetics of amikacin in preterm infants during the first days of life. Biol. Neonate 86:207-211. - PubMed
-
- Aust, G. 2001. Vestibulotoxicity and ototoxicity of gentamycin in newborns at risk. Int. Tinnitus J. 7:27-29. - PubMed
-
- Bonsu, B. K., and M. B. Harper. 2005. Accuracy and test characteristics of ancillary tests of cerebrospinal fluid for predicting acute bacterial meningitis in children with low white blood cell counts in cerebrospinal fluid. Acad. Emerg. Med. 12:303-309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

