Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate
- PMID: 18378716
- PMCID: PMC2415774
- DOI: 10.1128/AAC.00645-07
Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate
Abstract
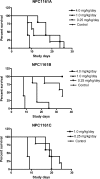
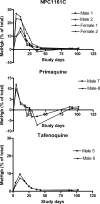
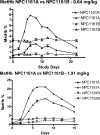
8-Aminoquinolines are an important class of antiparasitic agents, with broad utility and excellent efficacy, but also limitations due to hematological toxicities, primarily methemoglobinemia and hemolysis. One representative from this class, (+/-)-8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate (NPC1161C), proved extremely efficacious in animal models of malaria and pneumocystis pneumonia. This racemic mixture was separated into its component enantiomers by chemical and chromatographic means. The enantiomers were evaluated for antiparasitic activity in murine models of Plasmodium berghei, Pneumocystis carinii, and Leishmania donovani infection, as well as the propensity to elicit hematotoxicity in dogs. The (-)-enantiomer NPC1161B was found to be more active (by severalfold, depending on the dosing regimen) than the (+)-enantiomer NPC1161A in all of these murine models. In addition, the (-) enantiomer showed markedly reduced general toxicity in mice and reduced hematotoxicity in the dog model of methemoglobinemia. It is concluded that the configuration at the asymmetric center in the 8-amino side chain differentially affects efficacy and toxicity profiles and thus may be an important determinant of the "therapeutic window" for compounds in this class.
Figures

References
-
- Agarwal, S., U. R. Gupta, R. C. Gupta, N. Anand, and S. S. Agarwal. 1988. Susceptibility of glucose-6-phosphate dehydrogenase-deficient red cells to primaquine enantiomers and two putative metabolites. I. Effect on reduced glutathione, methemoglobin content and release of hemoglobin. Biochem. Pharmacol. 37:4605-4609. - PubMed
-
- Baird, J. K., and S. L. Hoffman. 2004. Primaquine therapy for malaria. Clin. Infect. Dis. 39:1336-1345. - PubMed
-
- Baird, J. K., G. J. McCormick, and C. J. Canfield. 1986. Effects of nine synthetic putative metabolites of primaquine on activity of the hexose monophosphate shunt in intact human red blood cells in vitro. Biochem. Pharmacol. 35:1099-1106. - PubMed
-
- Baker, J. K., and J. D. McChesney. 1988. Differential metabolism of the enantiomers of primaquine. J. Pharm. Sci. 77:380-382. - PubMed
-
- Bartlett, M. S., S. F. Queener, R. R. Tidwell, W. K. Milhous, J. D. Berman, W. Y. Ellis, and J. W. Smith. 1991. 8-Aminoquinolines from Walter Reed Army Institute for Research for treatment and prophylaxis of Pneumocystis pneumonia in rat models. Antimicrob. Agents Chemother. 35:277-282. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

