A minimal binding footprint on CD1d-glycolipid is a basis for selection of the unique human NKT TCR
- PMID: 18378792
- PMCID: PMC2292230
- DOI: 10.1084/jem.20072141
A minimal binding footprint on CD1d-glycolipid is a basis for selection of the unique human NKT TCR
Abstract
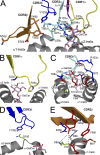
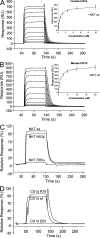
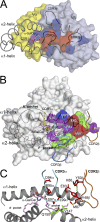
Although it has been established how CD1 binds a variety of lipid antigens (Ag), data are only now emerging that show how alphabeta T cell receptors (TCRs) interact with CD1-Ag. Using the structure of the human semiinvariant NKT TCR-CD1d-alpha-galactosylceramide (alpha-GalCer) complex as a guide, we undertook an alanine scanning mutagenesis approach to define the energetic basis of this interaction between the NKT TCR and CD1d. Moreover, we explored how analogues of alpha-GalCer affected this interaction. The data revealed that an identical energetic footprint underpinned the human and mouse NKT TCR-CD1d-alpha-GalCer cross-reactivity. Some, but not all, of the contact residues within the Jalpha18-encoded invariant CDR3alpha loop and Vbeta11-encoded CDR2beta loop were critical for recognizing CD1d. The residues within the Valpha24-encoded CDR1alpha and CDR3alpha loops that contacted the glycolipid Ag played a smaller energetic role compared with the NKT TCR residues that contacted CD1d. Collectively, our data reveal that the region distant to the protruding Ag and directly above the F' pocket of CD1d was the principal factor in the interaction with the NKT TCR. Accordingly, although the structural footprint at the NKT TCR-CD1d-alpha-GalCer is small, the energetic footprint is smaller still, and reveals the minimal requirements for CD1d restriction.
Figures


References
-
- Zinkernagel, R.M., and P.C. Doherty. 1997. The discovery of MHC restriction. Immunol. Today. 18:14–17. - PubMed
-
- Brigl, M., and M.B. Brenner. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22:817–890. - PubMed
-
- Ulrichs, T., and S.A. Porcelli. 2000. CD1 proteins: targets of T cell recognition in innate and adaptive immunity. Rev. Immunogenet. 2:416–432. - PubMed
-
- Moody, D.B., D.M. Zajonc, and I.A. Wilson. 2005. Anatomy of CD1-lipid antigen complexes. Nat. Rev. Immunol. 5:387–399. - PubMed
-
- Kobayashi, E., K. Motoki, T. Uchida, H. Fukushima, and Y. Koezuka. 1995. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol. Res. 7:529–534. - PubMed

