CXCR4 is required for the quiescence of primitive hematopoietic cells
- PMID: 18378795
- PMCID: PMC2292218
- DOI: 10.1084/jem.20072513
CXCR4 is required for the quiescence of primitive hematopoietic cells
Abstract
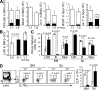
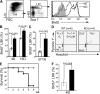
The quiescence of hematopoietic stem cells (HSCs) is critical for preserving a lifelong steady pool of HSCs to sustain the highly regenerative hematopoietic system. It is thought that specialized niches in which HSCs reside control the balance between HSC quiescence and self-renewal, yet little is known about the extrinsic signals provided by the niche and how these niche signals regulate such a balance. We report that CXCL12 produced by bone marrow (BM) stromal cells is not only the major chemoattractant for HSCs but also a regulatory factor that controls the quiescence of primitive hematopoietic cells. Addition of CXCL12 into the culture inhibits entry of primitive hematopoietic cells into the cell cycle, and inactivation of its receptor CXCR4 in HSCs causes excessive HSC proliferation. Notably, the hyperproliferative Cxcr4(-/-) HSCs are able to maintain a stable stem cell compartment and sustain hematopoiesis. Thus, we propose that CXCR4/CXCL12 signaling is essential to confine HSCs in the proper niche and controls their proliferation.
Figures
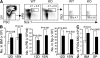

References
-
- Wilson, A., and A. Trumpp. 2006. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 6:93–106. - PubMed
-
- Cheng, T., N. Rodrigues, H. Shen, Y. Yang, D. Dombkowski, M. Sykes, and D.T. Scadden. 2000. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 287:1804–1808. - PubMed
-
- Hock, H., M.J. Hamblen, H.M. Rooke, J.W. Schindler, S. Saleque, Y. Fujiwara, and S.H. Orkin. 2004. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 431:1002–1007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

