Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum
- PMID: 18378904
- PMCID: PMC2291096
- DOI: 10.1073/pnas.0710389105
Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum
Abstract
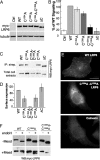
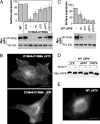
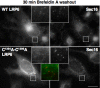
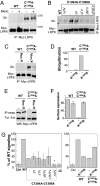
Canonical Wnt signaling is initiated by binding of Wnt proteins to members of the Frizzled family and subsequent complex formation with lipoprotein receptor-related proteins 5/6 (LRP5/6). Here, we show that LRP6 is palmitoylated on a juxtamembranous cysteine and that palmitoylation is required for exit from the endoplasmic reticulum (ER). We propose that palmitoylation serves to tilt the long, 23-residue transmembrane domain of LRP6 with respect to the plane of membrane to prevent a hydrophobic mismatch and subsequent recognition by the ER quality control. In support of this model, a palmitoylation-deficient LRP6 mutant could be rescued from ER retention by deletion of two to four residues in the transmembrane domain. Importantly, we found that palmitoylation-deficient LRP6 was retained in the ER by a completely novel monoubiquitination-dependent ER retention mechanism. Mutation of a specific lysine indeed abolished ubiquitination of palmitoylation-deficient LRP6 and led to a rescue from ER retention. Finally, at the cell surface, we found that interplay between palmitoylation and ubiquitination was necessary for efficient Wnt signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 1997;11:3286–3305. - PubMed
-
- Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. - PubMed
-
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
-
- Koduri V, Blacklow SC. Requirement for natively unstructured regions of mesoderm development candidate 2 in promoting low-density lipoprotein receptor-related protein 6 maturation. Biochemistry. 2007;46:6570–6577. - PubMed
-
- Hsieh JC, et al. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell. 2003;112:355–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

