Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus
- PMID: 18378908
- PMCID: PMC2291127
- DOI: 10.1073/pnas.0801388105
Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus
Abstract
In the absence of an effective vaccine, there is an urgent need for safe and effective antiviral agents to prevent transmission of HIV. Here, we report that an amphipathic alpha-helical peptide derived from the hepatitis C virus NS5A anchor domain (designated C5A in this article) that has been shown to be virocidal for the hepatitis C virus (HCV) also has potent antiviral activity against HIV. C5A exhibits a broad range of antiviral activity against HIV isolates, and it prevents infection of the three in vivo targets of HIV: CD4(+) T lymphocytes, macrophages, and dendritic cells by disrupting the integrity of the viral membrane and capsid core while preserving the integrity of host membranes. C5A can interrupt an ongoing T cell infection, and it can prevent transmigration of HIV through primary genital epithelial cells, infection of mucosal target cells and transfer from dendritic cells to T cells ex vivo, justifying future experiments to determine whether C5A can prevent HIV transmission in vivo.
Conflict of interest statement
Conflict of interest statement: F.V.C. has a financial interest in Viriome, Inc., which has licensing rights to the information provided in this article.
Figures



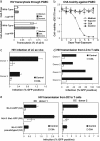
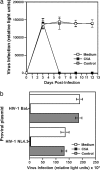
Similar articles
-
Antiviral activity of virocidal peptide derived from NS5A against two different HCV genotypes: an in vitro study.J Immunoassay Immunochem. 2015;36(1):63-79. doi: 10.1080/15321819.2014.896264. J Immunoassay Immunochem. 2015. PMID: 24606010
-
A virocidal amphipathic {alpha}-helical peptide that inhibits hepatitis C virus infection in vitro.Proc Natl Acad Sci U S A. 2008 Feb 26;105(8):3088-93. doi: 10.1073/pnas.0712380105. Epub 2008 Feb 19. Proc Natl Acad Sci U S A. 2008. PMID: 18287023 Free PMC article.
-
Syndecan-Fc hybrid molecule as a potent in vitro microbicidal anti-HIV-1 agent.Antimicrob Agents Chemother. 2010 Jul;54(7):2753-66. doi: 10.1128/AAC.01606-09. Epub 2010 May 3. Antimicrob Agents Chemother. 2010. PMID: 20439611 Free PMC article.
-
Impact of human immune deficiency virus infection on hepatitis C virus infection and replication.Curr HIV Res. 2007 Jan;5(1):55-67. doi: 10.2174/157016207779316341. Curr HIV Res. 2007. PMID: 17266557 Review.
-
Repression of the PKR protein kinase by the hepatitis C virus NS5A protein: a potential mechanism of interferon resistance.Clin Diagn Virol. 1998 Jul 15;10(2-3):157-62. doi: 10.1016/s0928-0197(98)00034-8. Clin Diagn Virol. 1998. PMID: 9741641 Review.
Cited by
-
Peptide-Based HIV Entry Inhibitors.Adv Exp Med Biol. 2022;1366:15-26. doi: 10.1007/978-981-16-8702-0_2. Adv Exp Med Biol. 2022. PMID: 35412132
-
Histidine-rich Modification of a Scorpion-derived Peptide Improves Bioavailability and Inhibitory Activity against HSV-1.Theranostics. 2018 Jan 1;8(1):199-211. doi: 10.7150/thno.21425. eCollection 2018. Theranostics. 2018. PMID: 29290802 Free PMC article.
-
A p7 Ion Channel-derived Peptide Inhibits Hepatitis C Virus Infection in Vitro.J Biol Chem. 2015 Sep 18;290(38):23254-63. doi: 10.1074/jbc.M115.662452. Epub 2015 Aug 6. J Biol Chem. 2015. PMID: 26251517 Free PMC article.
-
Molecular recognition of the native HIV-1 MPER revealed by STED microscopy of single virions.Nat Commun. 2019 Jan 8;10(1):78. doi: 10.1038/s41467-018-07962-9. Nat Commun. 2019. PMID: 30622256 Free PMC article.
-
Cyclophilin Inhibitors Remodel the Endoplasmic Reticulum of HCV-Infected Cells in a Unique Pattern Rendering Cells Impervious to a Reinfection.PLoS One. 2016 Jul 21;11(7):e0159511. doi: 10.1371/journal.pone.0159511. eCollection 2016. PLoS One. 2016. PMID: 27442520 Free PMC article.
References
-
- Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:291–307. - PubMed
-
- Chatterji U, et al. Naturally occurring capsid substitutions render HIV-1 cyclophilin A independent in human cells and TRIM-cyclophilin-resistant in Owl monkey cells. J Biol Chem. 2005;280:40293–40300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

