Expectations of benefit in early-phase clinical trials: implications for assessing the adequacy of informed consent
- PMID: 18378940
- PMCID: PMC2630499
- DOI: 10.1177/0272989X08315242
Expectations of benefit in early-phase clinical trials: implications for assessing the adequacy of informed consent
Abstract
Background: Participants in early-phase clinical trials have reported high expectations of benefit from their participation. There is concern that participants misunderstand the trials to which they have consented, which is based on assumptions about what patients mean when responding to questions about likelihood of benefit.
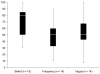
Methods: Participants were 27 women and 18 men in early-phase oncology trials at 2 academic medical centers in the United States. To determine whether expectations of benefit differ depending on how patients are queried, the authors randomly assigned participants to 1 of 3 interviews corresponding to 3 questions about likelihood of benefit: frequency type, belief type, and vague. In semistructured interviews, participants were queried about how they understood and answered the question. Participants then answered and discussed 1 of the other questions.
Results: Expectations of benefit in response to the belief-type question were significantly greater than expectations in response to the frequency-type and vague questions (P=0:02). The most common justifications involved positive attitude (n=27 [60%]) and references to physical health (n=23 [51%]). References to positive attitude were most common among participants with higher (> 70%) expectations (n = 11 [85%]) and least common among those with lower ( < 50%) expectations (n = 3 [27%]).
Conclusions: The wording of questions about likelihood of benefit shapes the expectations that patients express. Patients who express high expectations may not do so to communicate understanding but rather to register optimism. Ongoing research will clarify the meaning of high expectations and examine methods for assessing understanding.
Figures
References
-
- Agrawal M, Emanuel EJ. Ethics of phase 1 oncology studies: reexamining the arguments and the data. JAMA. 2003;290:1075–82. - PubMed
-
- Menikoff J. Consent forms for oncology trials. N Engl J Med. 2003;348:1496–7. - PubMed
-
- King NM, Henderson GE, Churchill LR, et al. Consent forms and the therapeutic misconception. IRB. 2005;27:1–8. - PubMed
-
- Henderson GE, Easter MM, Zimmer C, et al. Therapeutic misconception in early phase gene transfer trials. Soc Sci Med. 2006;62:239–53. - PubMed
-
- Horng S, Emanuel EJ, Wilfond B, Rackoff J, Martz K, Grady C. Descriptions of benefits and risks in consent forms for phase 1 oncology trials. N Engl J Med. 2002;347:2134–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

