Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease
- PMID: 18379437
- PMCID: PMC3243760
- DOI: 10.1097/NEN.0b013e31816a1df3
Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease
Abstract
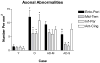
A large body of evidence indicates that basal forebrain cholinergic neurons are selectively vulnerable to degeneration early in Alzheimer disease (AD). Recent studies, however, demonstrate reductions in cortical activity of the cholinergic enzyme choline acetyltransferase only in late stages of AD. To address this apparent contradiction, we compared abnormalities in magnocellular basal forebrain cholinergic neurons and their axons in nondemented young (<65 years; n = 6), nondemented old (>65 years; n = 7), pathologically mild (n = 5), and pathologically severe (n = 5) AD cases. Cholinergic axon abnormalities (i.e. thickened fibers and ballooned terminals) were evident in nondemented middle-aged cases, increased in nondemented old cases, and reduced in density in severe AD. This suggests that loss of cortical cholinergic axons in AD occurs preferentially in fibers with these abnormalities. Paired helical filament 1-immunoreactive pretangles and tangles were observed as early as the third decade prior to their appearance in entorhinal/perirhinal cortex; they were increased in mild and severe AD. These results indicate that basal forebrain cholinergic neuron abnormalities are present very early in aging and in the course of AD. Therefore, despite the morphologic alterations, choline acetyltransferase activity, but not necessarily normal neuron functions, may be preserved.
Figures




References
-
- Arendash GW, Millard WJ, Dunn AJ, Meyer EM. Long-term neuropathological and neurochemical effects of nucleus basalis lesions in the rat. Science. 1987;238:952–6. - PubMed
-
- Arendt T, Bigl V, Tennstedt A, Arndt A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer’s disease. Neurosci. 1985;14:1–14. - PubMed
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex. 1991;1:103–16. - PubMed
-
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–8. - PubMed
-
- Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, Schmeidler J, Kanof P, Davis KL. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64:749–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

