Cryoradiolytic reduction of heme proteins: Maximizing dose dependent yield
- PMID: 18379640
- PMCID: PMC2131698
- DOI: 10.1016/j.radphyschem.2006.03.002
Cryoradiolytic reduction of heme proteins: Maximizing dose dependent yield
Abstract
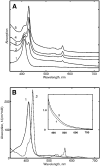
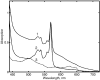
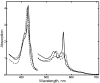
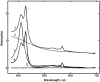
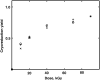
Radiolytic reduction in frozen solutions and crystals is a useful method for generation of trapped intermediates in protein based radical reactions. In this communication we define the conditions which provide the maximum yield of one electron reduced myoglobin at 77 K using (60)Co γ-irradiation in aqueous glycerol glass. The yield reached 50% after 20 kGy, was almost complete at ∼160 kGy total dose, and does not depend on the protein concentration in the range 0.01 - 5 mM.
Figures
Similar articles
-
Electron paramagnetic resonance and electron-nuclear double resonance studies of the reactions of cryogenerated hydroperoxoferric-hemoprotein intermediates.Biochemistry. 2014 Aug 5;53(30):4894-903. doi: 10.1021/bi500296d. Epub 2014 Jul 21. Biochemistry. 2014. PMID: 25046203 Free PMC article.
-
Cryoradiolytic reduction of crystalline heme proteins: analysis by UV-Vis spectroscopy and X-ray crystallography.J Synchrotron Radiat. 2007 Jan;14(Pt 1):11-23. doi: 10.1107/S0909049506049806. Epub 2006 Dec 15. J Synchrotron Radiat. 2007. PMID: 17211068
-
Radiolytic oxidation and degradation of 2,4-dichlorophenol in aqueous solutions.Environ Sci Pollut Res Int. 2019 Jun;26(17):17055-17065. doi: 10.1007/s11356-019-04845-4. Epub 2019 Apr 17. Environ Sci Pollut Res Int. 2019. PMID: 30997644
-
Primary and secondary sludge treatment using ionizing radiation technology in Alexandria, Egypt.Appl Radiat Isot. 2022 Mar;181:110101. doi: 10.1016/j.apradiso.2022.110101. Epub 2022 Jan 10. Appl Radiat Isot. 2022. PMID: 35065517 Review.
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
Cited by
-
Cryoradiolysis and cryospectroscopy for studies of heme-oxygen intermediates in cytochromes p450.Methods Mol Biol. 2012;875:375-91. doi: 10.1007/978-1-61779-806-1_20. Methods Mol Biol. 2012. PMID: 22573452 Free PMC article.
-
Dose-resolved serial synchrotron and XFEL structures of radiation-sensitive metalloproteins.IUCrJ. 2019 May 3;6(Pt 4):543-551. doi: 10.1107/S2052252519003956. eCollection 2019 Jul 1. IUCrJ. 2019. PMID: 31316799 Free PMC article.
-
Combined microspectrophotometric and crystallographic examination of chemically reduced and X-ray radiation-reduced forms of cytochrome ba3 oxidase from Thermus thermophilus: structure of the reduced form of the enzyme.Biochemistry. 2009 Feb 10;48(5):820-6. doi: 10.1021/bi801759a. Biochemistry. 2009. PMID: 19140675 Free PMC article.
-
The critical iron-oxygen intermediate in human aromatase.Biochem Biophys Res Commun. 2009 Sep 11;387(1):169-73. doi: 10.1016/j.bbrc.2009.06.154. Epub 2009 Jul 8. Biochem Biophys Res Commun. 2009. PMID: 19591804 Free PMC article.
-
The ferric-hydroperoxo complex of chloroperoxidase.Biochem Biophys Res Commun. 2007 Nov 30;363(4):954-8. doi: 10.1016/j.bbrc.2007.09.085. Epub 2007 Oct 1. Biochem Biophys Res Commun. 2007. PMID: 17920039 Free PMC article.
References
-
- Antonini E, Brunori M. Hemoglobin and myoglobin in their reactions with ligands. Amsterdam: North Holland Publishing Co.; 1971.
-
- Ausloos P, editor. Fundamental Processes in Radiation Chemistry. New York: Wiley Interscience Publishers; 1968.
-
- Berglund GI, Carlsson GH, Smith AT, Szoeke H, Henriksen A, Hajdu J. The catalytic pathway of horseradish peroxidase at high resolution. Nature. 2002;417(6887):463–468. - PubMed
-
- Davydov R, Kofman V, Fujii H, Yoshida T, Ikeda-Saito M, Hoffman BM. Catalytic Mechanism of Heme Oxygenase through EPR and ENDOR of Cryoreduced Oxy-Heme Oxygenase and Its Asp 140 Mutants. J Amer Chem Soc. 2002;124(8):1798–1808. - PubMed
-
- Davydov R, Kuprin S, Graeslund A, Ehrenberg A. Electron Paramagnetic Resonance Study of the Mixed-Valent Diiron Center in Escherichia coli Ribonucleotide Reductase Produced by Reduction of Radical-Free Protein R2 at 77 K. J Amer Chem Soc. 1994;116(24):11120–11128.
Grants and funding
LinkOut - more resources
Full Text Sources
