Development of thermotolerance requires interaction between polymerase-beta and heat shock proteins
- PMID: 18380790
- PMCID: PMC11159698
- DOI: 10.1111/j.1349-7006.2008.00759.x
Development of thermotolerance requires interaction between polymerase-beta and heat shock proteins
Abstract
Although heat shock proteins (HSP) are well known to contribute to thermotolerance, they only play a supporting role in the phenomenon. Recently, it has been reported that heat sensitivity depends on heat-induced DNA double-strand breaks (DSB), and that thermotolerance also depends on the suppression of DSB formation. However the critical elements involved in thermotolerance have not yet been fully identified. Heat produces DSB and leads to cell death through denaturation and dysfunction of heat-labile repair proteins such as DNA polymerase-beta (Pol beta). Here the authors show that thermotolerance was partially suppressed in Pol beta(-/-) mouse embryonic fibroblasts (MEF) when compared to the wild-type MEF, and was also suppressed in the presence of the HSP inhibitor, KNK437, in both cell lines. Moreover, the authors found that heat-induced gamma H2AX was suppressed in the thermotolerant cells. These results suggest that Pol beta at least contributes to thermotolerance through its reactivation and stimulation by Hsp27 and Hsp70. In addition, it appears possible that fewer DSB were formed after a challenging heat exposure because preheat-induced Hsp27 and Hsp70 can rescue or restore other, as yet unidentified, heat-labile proteins besides Pol beta. The present novel findings provide strong evidence that Pol beta functions as a critical element involved in thermotolerance and exerts an important role in heat-induced DSB.
Figures
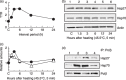
 ) Hsp27. (
) Hsp27. ( ) Hsp70. (d) Co‐immunoprecipitation experiments with nuclear extracts. Lane 1, non‐heating treatment; lane 2, 3 h after a preheating treatment alone (45.5°C, 5 min); lane 3, challenging heat treatment (45.5°C, 20 min) at 3 h after a preheating treatment (45.5°C, 5 min); lane 4, challenging heat treatment alone (45.5°C, 20 min).
) Hsp70. (d) Co‐immunoprecipitation experiments with nuclear extracts. Lane 1, non‐heating treatment; lane 2, 3 h after a preheating treatment alone (45.5°C, 5 min); lane 3, challenging heat treatment (45.5°C, 20 min) at 3 h after a preheating treatment (45.5°C, 5 min); lane 4, challenging heat treatment alone (45.5°C, 20 min).
 ) Polβ+/+ MEFs. (
) Polβ+/+ MEFs. ( ) Polβ−/– MEFs. *P < 0.05 and **P < 0.01, by Student's t‐test between Polβ+/+ and Polβ−/– MEF, respectively.
) Polβ−/– MEFs. *P < 0.05 and **P < 0.01, by Student's t‐test between Polβ+/+ and Polβ−/– MEF, respectively.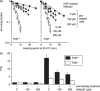
 ,
, ,
, ) Cells were not conditioned by a preheating treatment. (
) Cells were not conditioned by a preheating treatment. ( ,
, ,
, ) Cells were conditioned by a preheating treatment. Left panel: Polβ+/+ MEF. Right panel: Polβ−/– MEF. (
) Cells were conditioned by a preheating treatment. Left panel: Polβ+/+ MEF. Right panel: Polβ−/– MEF. ( ,
, ) 0 µM KNK437. (
) 0 µM KNK437. ( ,
, ) 100 µM KNK437. (
) 100 µM KNK437. ( ,
, ) 300 µM KNK437. (b) Thermotolerance ratios. (
) 300 µM KNK437. (b) Thermotolerance ratios. ( ) Polβ+/+ MEF. (
) Polβ+/+ MEF. ( ) Polβ−/– MEF.
) Polβ−/– MEF.
 ) Polβ+/+ MEF. (
) Polβ+/+ MEF. ( ) Polβ−/– MEF.
) Polβ−/– MEF.
References
-
- Ohnishi K, Ohnishi T. Heat‐induced p53‐dependent signal transduction and its role in hyperthermic cancer therapy. Int J Hyperthermia 2001; 17: 415–27. - PubMed
-
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol 2001; 77: 399–408. - PubMed
-
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994; 10: 457–83. - PubMed
-
- Nakahata K, Miyakoda M, Suzuki K, Kodama S, Watanabe M. Heat shock induces centrosomal dysfunction, and causes non‐apoptotic mitotic catastrophe in human tumour cells. Int J Hyperthermia 2002; 18: 332–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

