Downregulation of Tie2 gene by a novel antitumor sulfolipid, 3'-sulfoquinovosyl-1'-monoacylglycerol, targeting angiogenesis
- PMID: 18380795
- PMCID: PMC11158498
- DOI: 10.1111/j.1349-7006.2008.00785.x
Downregulation of Tie2 gene by a novel antitumor sulfolipid, 3'-sulfoquinovosyl-1'-monoacylglycerol, targeting angiogenesis
Abstract
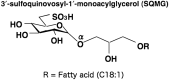
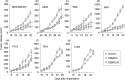
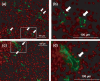
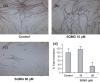
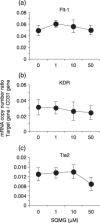
We previously reported that 3'-sulfoquinovosyl-1'-monoacylglycerol (SQMG) was effective in suppressing the growth of solid tumors due to hemorrhagic necrosis in vivo. In the present study, we investigated the antiangiogenic effect of SQMG. In vivo assessment of antitumor assays showed that some tumor cell lines, but not others, were sensitive to SQMG. Microscopic study suggested that in SQMG-sensitive tumors, but not SQMG-resistant tumors, angiogenesis was reduced. We next investigated gene expression relating to angiogenesis in tumor tissues by quantitative real-time polymerase chain reaction. Consequently, although vascular endothelial growth factor gene expression was not detected with significant differences among the cases, significant downregulation of Tie2 gene expression was observed in all SQMG-sensitive tumors as compared with controls, but not in SQMG-resistant tumors. These data suggested that the antitumor effects of SQMG could be attributed to antiangiogenic effects, possibly via the downregulation of Tie2 gene expression in SQMG-sensitive tumors.
Figures

References
-
- Folkman J. What is the evidence that tumor are angiogenesis dependent? J Natl Cancer Inst 1990; 82: 4–6. - PubMed
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature 2005; 438: 932–6. - PubMed
-
- O’Reilly MS, Holmgren L, Shing Y et al . Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79: 315–28. - PubMed
-
- O’Reilly MS, Boehm T, Shing Y et al . Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88: 277–85. - PubMed
-
- Fong TA, Shawver LK, Sun L et al . SU5416 is a potentand selective inhibitor of vascular endothelial growth factor receptor (Flk‐1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 1999; 59: 99–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

