Co-infection of respiratory bacterium with severe acute respiratory syndrome coronavirus induces an exacerbated pneumonia in mice
- PMID: 18380809
- PMCID: PMC7168413
- DOI: 10.1111/j.1348-0421.2008.00011.x
Co-infection of respiratory bacterium with severe acute respiratory syndrome coronavirus induces an exacerbated pneumonia in mice
Abstract
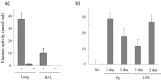
SARS-CoV grows in a variety of tissues that express its receptor, although the mechanism for high replication in the lungs and severe respiratory illness is not well understood. We recently showed that elastase enhances SARS-CoV infection in cultured cells, which suggests that SARS development may be due to elastase-mediated, enhanced SARS-CoV infection in the lungs. To explore this possibility, we examined whether co-infection of mice with SARS-CoV and Pp, a low-pathogenic bacterium which elicits elastase production in the lungs, induces exacerbation of pneumonia. Mice co-infected with SARS-CoV and Pp developed severe respiratory disease with extensive weight loss, resulting in a 33~90% mortality rate. Mice with exacerbated pneumonia showed enhanced virus infection in the lungs and histopathological lesions similar to those found in human SARS cases. Intranasal administration of LPS, another elastase inducer, showed an effect similar to that of Pp infection. Thus, this study shows that exacerbated pneumonia in mice results from co-infection with SARS-CoV and a respiratory bacterium that induces elastase production in the lungs, suggesting a possible role for elastase in the exacerbation of pneumonia.
Figures


References
-
- Drosten C., Gunther S., Preiser W., Van Der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikowa L., Fouchier R.A., Berger A., Burguiere A.M., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Eng J Med 348: 1967–76. - PubMed
-
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., Van Amerongen G., Van Riel D., Laman J.D., De Jong T., Van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., Van Der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S., Osterhaus A.D. (2003) Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362: 263–70. - PMC - PubMed
-
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks‐Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. (2003) The genome sequence of SARS‐associated coronavirus. Science 300: 1399–404. - PubMed
-
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen‐Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. (2003) Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300: 1394–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

