Functional surfaces on the p35/ARPC2 subunit of Arp2/3 complex required for cell growth, actin nucleation, and endocytosis
- PMID: 18381280
- PMCID: PMC2423265
- DOI: 10.1074/jbc.M800783200
Functional surfaces on the p35/ARPC2 subunit of Arp2/3 complex required for cell growth, actin nucleation, and endocytosis
Abstract
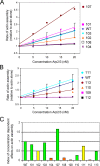
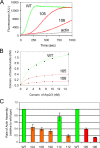
The Arp2/3 complex is comprised of seven evolutionarily conserved subunits and upon activation by WASp or another nucleation promoting factor nucleates the formation of actin filaments. These events are critical for driving a wide range of cellular processes, including motility, endocytosis, and intracellular trafficking. However, an in depth understanding of the Arp2/3 complex activation and nucleation mechanism is still lacking. Here, we used a mutagenesis approach in Saccharomyces cerevisiae to dissect the structural and functional roles of the p35/ARPC2 subunit. Using integrated alleles that target conserved and solvent-exposed residues, we identified surfaces on p35/ARPC2 required for cell growth, actin organization, and endocytosis. In parallel, we purified the mutant Arp2/3 complexes and compared their actin assembly activities both in the presence and in the absence of WASp. The majority of alleles with defects mapped to one face of p35/ARPC2, where there was a close correlation between loss of actin nucleation and endocytosis. A second site required for nucleation and endocytosis was identified near the contact surface between p35/ARPC2 and p19/ARPC4. A third site was identified at a more distal conserved surface, which was critical for endocytosis but not nucleation. These findings pinpoint the key surfaces on p35/ARPC2 required for Arp2/3 complex-mediated actin assembly and cellular function and provide a higher resolution view of Arp2/3 structure and mechanism.
Figures





References
-
- Goley, E. D., and Welch, M. D. (2006) Nat. Rev. Mol. Cell. Biol. 7 713-726 - PubMed
-
- Robinson, R. C., Turbedsky, K., Kaiser, D. A., Marchand, J. B., Higgs, H. N., Choe, S., and Pollard, T. D. (2001) Science 294 1679-1684 - PubMed
-
- Welch, M. D., and Mullins, R. D. (2002) Annu. Rev. Cell Dev. Biol. 18 247-288 - PubMed
-
- Goley, E. D., Rodenbusch, S. E., Martin, A. C., and Welch, M. D. (2004) Mol. Cell 16 269-279 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

