Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons
- PMID: 18381284
- PMCID: PMC2414263
- DOI: 10.1074/jbc.M801553200
Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons
Abstract
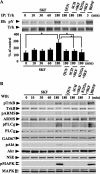
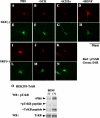
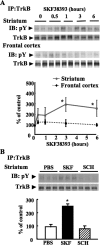
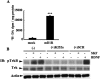
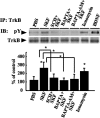
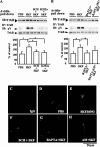
In addition to its role as a neurotransmitter, dopamine can stimulate neurite outgrowth and morphological effects upon primary neurons. To investigate the signal transduction mechanisms used by dopamine in developing striatal neurons, we focused upon the effects of activating the dopamine D1 receptor. Using the D1 receptor agonist SKF38393, we found that Trk neurotrophin receptors were activated in embryonic day 18 striatal neurons. K-252a, a Trk tyrosine kinase inhibitor, and a dopamine D1 receptor antagonist could block the effects of SKF38393. The increase in TrkB phosphorylation was not the result of increased neurotrophin production. Induction of TrkB activity by SKF38393 was accompanied by the phosphorylation of several Trk signaling proteins, including phospholipase Cgamma, Akt, and MAPK. Biotinylation experiments followed by immunostaining by phospho-TrkB-specific antibodies indicated that the mechanism involved increased TrkB surface expression by dopamine D1 receptor activation. This increase in cell surface TrkB expression was dependent upon an increase in intracellular Ca(2+). These results indicate that stimulation of dopamine D1 receptors can be coupled to the neurotrophin receptor signaling to mediate the effects of dopamine upon striatal neurons.
Figures

References
-
- Kalivas, P. W. (1993) Brain Res. 18 75-113 - PubMed
-
- Missale, C., Nash, S. R., Robinson, S. W., Jaber, M., and Caron, M. G. (1998) Physiol. Rev. 78 189-225 - PubMed
-
- Bustos, G., Abarca, J., Campusano, J., Bustos, V., Noriega, V., and Aliaga, E. (2004) Brain Res. 47 126-144 - PubMed
-
- Xu, M., Hu, X. T., Cooper, D. C., Moratalla, R., Graybiel, A. M., White, F. J., and Tonegawa, S. (1994) Cell 79 945-955 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

