Bioluminescence resonance energy transfer assays reveal ligand-specific conformational changes within preformed signaling complexes containing delta-opioid receptors and heterotrimeric G proteins
- PMID: 18381293
- PMCID: PMC3258885
- DOI: 10.1074/jbc.M707941200
Bioluminescence resonance energy transfer assays reveal ligand-specific conformational changes within preformed signaling complexes containing delta-opioid receptors and heterotrimeric G proteins
Abstract
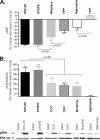
Heptahelical receptors communicate extracellular information to the cytosolic compartment by binding an extensive variety of ligands. They do so through conformational changes that propagate to intracellular signaling partners as the receptor switches from a resting to an active conformation. This active state has been classically considered unique and responsible for regulation of all signaling pathways controlled by a receptor. However, recent functional studies have challenged this notion and called for a paradigm where receptors would exist in more than one signaling conformation. This study used bioluminescence resonance energy transfer assays in combination with ligands of different functional profiles to provide in vivo physical evidence of conformational diversity of delta-opioid receptors (DORs). DORs and alpha(i1)beta(1)gamma(2) G protein subunits were tagged with Luc or green fluorescent protein to produce bioluminescence resonance energy transfer pairs that allowed monitoring DOR-G protein interactions from different vantage points. Results showed that DORs and heterotrimeric G proteins formed a constitutive complex that underwent structural reorganization upon ligand binding. Conformational rearrangements could not be explained by a two-state model, supporting the idea that DORs adopt ligand-specific conformations. In addition, conformational diversity encoded by the receptor was conveyed to the interaction among heterotrimeric subunits. The existence of multiple active receptor states has implications for the way we conceive specificity of signal transduction.
Figures
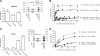




References
-
- Kenakin, T. (2004) Trends Pharmacol. Sci. 25 186–192 - PubMed
-
- Pineyro, G., and Archer-Lahlou, E. (2007) Cell. Signal. 19 8–19 - PubMed
-
- Urban, J. D., Clarke, W. P., von Zastrow, M., Nichols, D. E., Kobilka, B., Weinstein, H., Javitch, J. A., Roth, B. L., Christopoulos, A., Sexton, P. M., Miller, K. J., Spedding, M., and Mailman, R. B. (2007) J. Pharmacol. Exp. Ther. 320 1–13 - PubMed
-
- Berg, K. A., Maayani, S., Goldfarb, J., Scaramellini, C., Leff, P., and Clarke, W. P. (1998) Mol. Pharmacol. 54 94–104 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

