Phosphatase and tensin homologue deleted on chromosome 10 deficiency accelerates tumor induction in a mouse model of ErbB-2 mammary tumorigenesis
- PMID: 18381417
- PMCID: PMC2752841
- DOI: 10.1158/0008-5472.CAN-07-5727
Phosphatase and tensin homologue deleted on chromosome 10 deficiency accelerates tumor induction in a mouse model of ErbB-2 mammary tumorigenesis
Abstract
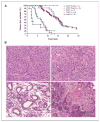
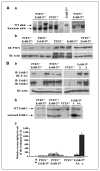
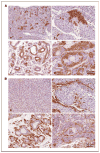
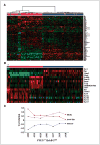
Loss of the tumor suppressor phosphatase and tensin homologue deleted on chromosome 10 (PTEN) and amplification or elevated expression of ErbB-2 are both involved in human breast cancer. To directly test the importance of these genetic events in mammary tumorigenesis, we have assessed whether mammary-specific disruption of PTEN could cooperate with activation of ErbB-2. Transgenic mice expressing ErbB-2 under the transcriptional control of its endogenous promoter (ErbB-2(KI)) were interbred with mice carrying conditional PTEN alleles and an MMTV/Cre transgene. Loss of one or both PTEN alleles resulted in a dramatic acceleration of mammary tumor onset and an increased occurrence of lung metastases in the ErbB-2(KI) strain. Tumor progression in PTEN-deficient/ErbB-2(KI) strains was associated with elevated ErbB-2 protein levels, which were not due to ErbB-2 amplification or to a dramatic increase in ErbB-2 transcripts. Moreover, the PTEN-deficient/ErbB-2(KI)-derived mouse mammary tumors display striking morphologic heterogeneity in comparison with the homogeneous pathology of the ErbB-2(KI) parental strain. Therefore, inactivation of PTEN would not only have a dramatic effect on ErbB-2-induced mammary tumorigenesis but would also lead to the formation of mammary tumors that, in part, display pathologic and molecular features associated with the basal-like subtype of primary human breast cancer.
Figures

References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Andrulis IL, Bull SB, Blackstein ME, et al. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol. 1998;16:1340–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

