Elevated ornithine decarboxylase levels activate ataxia telangiectasia mutated-DNA damage signaling in normal keratinocytes
- PMID: 18381427
- PMCID: PMC2392890
- DOI: 10.1158/0008-5472.CAN-07-5030
Elevated ornithine decarboxylase levels activate ataxia telangiectasia mutated-DNA damage signaling in normal keratinocytes
Abstract
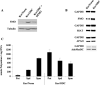
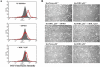
We examined the effect of increased expression of ornithine decarboxylase (ODC), a key rate-limiting enzyme in polyamine biosynthesis, on cell survival in primary cultures of keratinocytes isolated from the skin of K6/ODC transgenic mice (Ker/ODC) and their normal littermates (Ker/Norm). Although elevated levels of ODC and polyamines stimulate proliferation of keratinocytes, Ker/ODC undergo apoptotic cell death within days of primary culture unlike Ker/Norm that continue to proliferate. Phosphorylation of ataxia telangiectasia mutated (ATM) and its substrate p53 are significantly induced both in Ker/ODC and in K6/ODC transgenic skin. Chromatin immunoprecipitation analyses show that the increased level of p53 in Ker/ODC is accompanied by increased recruitment of p53 to the Bax proximal promoter. ATM activation is polyamine dependent because alpha-difluoromethylornithine, a specific inhibitor of ODC activity, blocks its phosphorylation. Ker/ODC also displays increased generation of H(2)O(2), acrolein-lysine conjugates, and protein oxidation products as well as polyamine-dependent DNA damage, as measured by the comet assay and the expression of the phosphorylated form of the histone variant gamma H2AX. Both reactive oxygen species generation and apoptotic cell death of Ker/ODC may, at least in part, be due to induction of a polyamine catabolic pathway that generates both H(2)O(2) and cytotoxic aldehydes, because spermine oxidase (SMO) levels are induced in Ker/ODC. In addition, treatment with MDL 72,527, an inhibitor of SMO, blocks the production of H(2)O(2) and increases the survival of Ker/ODC. These results show a novel activation of the ATM-DNA damage signaling pathway in response to increased ODC activity in nontumorigenic keratinocytes.
Figures




Similar articles
-
Elevated ornithine decarboxylase activity promotes skin tumorigenesis by stimulating the recruitment of bulge stem cells but not via toxic polyamine catabolic metabolites.Amino Acids. 2014 Mar;46(3):543-52. doi: 10.1007/s00726-013-1559-0. Epub 2013 Jul 25. Amino Acids. 2014. PMID: 23884694 Free PMC article.
-
Effect of elevated levels of ornithine decarboxylase on cell cycle progression in skin.Cell Growth Differ. 1999 Nov;10(11):739-48. Cell Growth Differ. 1999. PMID: 10593650
-
Ornithine decarboxylase expression leads to translocation and activation of protein kinase CK2 in vivo.J Biol Chem. 1997 May 9;272(19):12536-43. doi: 10.1074/jbc.272.19.12536. J Biol Chem. 1997. PMID: 9139705
-
Deregulation of polyamine biosynthesis alters intrinsic histone acetyltransferase and deacetylase activities in murine skin and tumors.Cancer Res. 2002 Jan 1;62(1):67-74. Cancer Res. 2002. PMID: 11782361
-
Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants.Cell Cycle. 2006 Sep;5(17):1940-5. doi: 10.4161/cc.5.17.3191. Epub 2006 Sep 1. Cell Cycle. 2006. PMID: 16940754 Free PMC article. Review.
Cited by
-
Polyamines and Their Metabolism: From the Maintenance of Physiological Homeostasis to the Mediation of Disease.Med Sci (Basel). 2022 Jul 15;10(3):38. doi: 10.3390/medsci10030038. Med Sci (Basel). 2022. PMID: 35893120 Free PMC article. Review.
-
Elevated epidermal ornithine decarboxylase activity suppresses contact hypersensitivity.J Invest Dermatol. 2011 Jan;131(1):158-66. doi: 10.1038/jid.2010.263. Epub 2010 Sep 16. J Invest Dermatol. 2011. PMID: 20844550 Free PMC article.
-
A prolonged and exaggerated wound response with elevated ODC activity mimics early tumor development.Carcinogenesis. 2011 Sep;32(9):1340-8. doi: 10.1093/carcin/bgr129. Epub 2011 Jul 5. Carcinogenesis. 2011. PMID: 21730362 Free PMC article.
-
Mechanisms of Mycotoxin-induced Dermal Toxicity and Tumorigenesis Through Oxidative Stress-related Pathways.J Toxicol Pathol. 2014 Apr;27(1):1-10. doi: 10.1293/tox.2013-0062. Epub 2014 Apr 30. J Toxicol Pathol. 2014. PMID: 24791061 Free PMC article. Review.
-
Elevated ornithine decarboxylase activity promotes skin tumorigenesis by stimulating the recruitment of bulge stem cells but not via toxic polyamine catabolic metabolites.Amino Acids. 2014 Mar;46(3):543-52. doi: 10.1007/s00726-013-1559-0. Epub 2013 Jul 25. Amino Acids. 2014. PMID: 23884694 Free PMC article.
References
-
- Gilmour SK, Birchler M, Smith MK, Rayca K, Mostochuk J. Effect of elevated levels of ornithine decarboxylase on cell cycle progression in skin. Cell Growth Differ. 1999;10:739–48. - PubMed
-
- O'Brien TG, Megosh LC, Gilliard G, Soler AP. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res. 1997;57(13):2630–7. - PubMed
-
- Smith MK, Trempus CS, Gilmour SK. Co-operation between follicular ornithine decarboxylase and v-Ha-ras induces spontaneous papillomas and malignant conversion in transgenic skin. Carcinogenesis. 1998;19(8):1409–15. - PubMed
-
- Ray RM, Zimmerman BJ, McCormack SA, Patel TB, Johnson LR. Polyamine depletion arrests cell cycle and induces inhibitors p21(Waf1/Cip1), p27(Kip1), and p53 in IEC-6 cells. Am J Physiol. 1999;276(3 Pt 1):C684–C91. - PubMed
-
- Kramer DL, Vujcic S, Diegelman P, et al. Polyamine analogue induction of the p53-p21WAF1/CIP1-Rb pathway and G1 arrest in human melanoma cells. Cancer Res. 1999;59(6):1278–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

