The role of amphiregulin in exemestane-resistant breast cancer cells: evidence of an autocrine loop
- PMID: 18381432
- PMCID: PMC2735892
- DOI: 10.1158/0008-5472.CAN-07-5544
The role of amphiregulin in exemestane-resistant breast cancer cells: evidence of an autocrine loop
Abstract
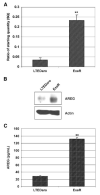
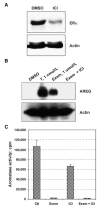
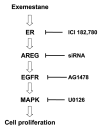
Exemestane-resistant breast cancer cell lines (i.e., ExeR), derived from MCF-7 cells expressing a high level of aromatase (MCF-7aro), were generated in our laboratory. The epidermal growth factor (EGF)-like protein amphiregulin (AREG) was highly expressed in ExeR cells based on cDNA microarray analysis. The high levels of AREG mRNA in ExeR cell lines were confirmed by real-time reverse transcription-PCR. The high levels of AREG protein in ExeR cell lysates and culture media were confirmed by Western blot analysis and ELISA, respectively. Furthermore, our Western blot analysis showed that whereas no AREG was detected in the DMSO control, overnight treatment of parental MCF-7aro cells with 1 micromol/L exemestane strongly induced the expression of AREG. This induction was totally blocked by 100 nmol/L of pure antiestrogen ICI 182,780, implying estrogen receptor (ER) dependence of exemestane-induced AREG expression. MCF-7aro cells were not able to proliferate in hormone-free medium, but were able to proliferate in conditioned medium from ExeR cells, similar to the treatment of recombinant human AREG. Small interference RNA targeting AREG inhibited ExeR proliferation, confirming that AREG is truly functioning as a growth factor of ExeR cells. The specific inhibitors to ER (ICI 182,780), EGF receptor (EGFR; AG1478), and mitogen-activated protein kinase (MAPK; U0126) all showed dose-dependent suppression of the proliferation of ExeR cells, indicating the involvement of the ER, EGFR, and MAPK pathways. Based on these findings, we propose a possible mechanism that underlies exemestane resistance: exemestane induces AREG in an ER-dependent manner. AREG then activates the EGFR pathway and leads to the activation of the MAPK pathway that drives cell proliferation.
Figures



Similar articles
-
Cross-suppression of EGFR ligands amphiregulin and epiregulin and de-repression of FGFR3 signalling contribute to cetuximab resistance in wild-type KRAS tumour cells.Br J Cancer. 2012 Apr 10;106(8):1406-14. doi: 10.1038/bjc.2012.103. Br J Cancer. 2012. PMID: 22491422 Free PMC article.
-
ADAM12 induces estrogen-independence in breast cancer cells.Breast Cancer Res Treat. 2012 Feb;131(3):731-41. doi: 10.1007/s10549-011-1431-4. Epub 2011 Mar 9. Breast Cancer Res Treat. 2012. PMID: 21387162 Free PMC article.
-
Knock-down of amphiregulin inhibits cellular invasion in inflammatory breast cancer.J Cell Physiol. 2011 Oct;226(10):2691-701. doi: 10.1002/jcp.22620. J Cell Physiol. 2011. PMID: 21302279 Free PMC article.
-
The ADAM17-amphiregulin-EGFR axis in mammary development and cancer.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):181-94. doi: 10.1007/s10911-008-9084-6. Epub 2008 May 10. J Mammary Gland Biol Neoplasia. 2008. PMID: 18470483 Free PMC article. Review.
-
Amphiregulin as a novel target for breast cancer therapy.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):171-9. doi: 10.1007/s10911-008-9081-9. Epub 2008 Apr 25. J Mammary Gland Biol Neoplasia. 2008. PMID: 18437539 Review.
Cited by
-
Mechanisms of resistance to endocrine therapy in breast cancer: focus on signaling pathways, miRNAs and genetically based resistance.Int J Mol Sci. 2012 Dec 20;14(1):108-45. doi: 10.3390/ijms14010108. Int J Mol Sci. 2012. PMID: 23344024 Free PMC article. Review.
-
An Exemestane Derivative, Oxymestane-D1, as a New Multi-Target Steroidal Aromatase Inhibitor for Estrogen Receptor-Positive (ER+) Breast Cancer: Effects on Sensitive and Resistant Cell Lines.Molecules. 2023 Jan 12;28(2):789. doi: 10.3390/molecules28020789. Molecules. 2023. PMID: 36677847 Free PMC article.
-
CITED1 as a marker of favourable outcome in anti-endocrine treated, estrogen-receptor positive, lymph-node negative breast cancer.BMC Res Notes. 2023 Jun 15;16(1):105. doi: 10.1186/s13104-023-06376-1. BMC Res Notes. 2023. PMID: 37322548 Free PMC article.
-
Valproic acid-induced amphiregulin secretion confers resistance to temozolomide treatment in human glioma cells.BMC Cancer. 2019 Aug 1;19(1):756. doi: 10.1186/s12885-019-5843-6. BMC Cancer. 2019. PMID: 31370819 Free PMC article.
-
Amphiregulin Is a Critical Downstream Effector of Estrogen Signaling in ERα-Positive Breast Cancer.Cancer Res. 2015 Nov 15;75(22):4830-8. doi: 10.1158/0008-5472.CAN-15-0709. Epub 2015 Nov 2. Cancer Res. 2015. PMID: 26527289 Free PMC article.
References
-
- Altundag K, Ibrahim NK. Aromatase inhibitors in breast cancer: an overview. Oncologist. 2006;11:553–62. - PubMed
-
- Brueggemeier RW, Hackett JC, Diaz-Cruz ES. Aromatase inhibitors in the treatment of breast cancer. Endocr Rev. 2005;26:331–45. - PubMed
-
- Wang X, Chen S. Aromatase destabilizer: novel action of exemestane, a Food and Drug Administration-approved aromatase inhibitor. Cancer Res. 2006;66:10281–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

