An Adam15 amplification loop promotes vascular endothelial growth factor-induced ocular neovascularization
- PMID: 18381816
- PMCID: PMC2493454
- DOI: 10.1096/fj.07-099283
An Adam15 amplification loop promotes vascular endothelial growth factor-induced ocular neovascularization
Abstract
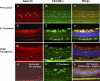
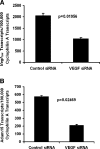
Proteins with a disintegrin and a metalloproteinase domain (ADAMs) are a family of membrane-bound proteinases that bind integrins through their disintegrin domain. In this study, we have found modest expression of ADAM15 in pericytes in normal retina and strong up-regulation of ADAM15 in retinal vascular endothelial cells in ischemic retina. Increased expression of vascular endothelial growth factor (VEGF) in the retina in the absence of ischemia also increased ADAM15 levels, and knockdown of Vegf mRNA in ischemic retina reduced Adam15 mRNA. Mice deficient in ADAM15 showed a significant reduction in ischemia-induced retinal neovascularization, choroidal neovascularization at rupture sites in Bruch's membrane, and VEGF-induced subretinal neovascularization. ADAM15-deficient mice also showed reduced levels of VEGF(164), VEGF receptor 1, and VEGF receptor 2 in ischemic retina. These data suggest that ADAM15 and VEGF participate in an amplification loop; VEGF increases expression of ADAM15, which in turn increases expression of VEGF and its receptors. Perturbation of the loop by elimination of ADAM15 suppresses ocular neovascularization in 3 different model systems, and thus ADAM15 provides a new therapeutic target for diseases complicated by neovascularization.
Figures
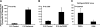




Similar articles
-
Implication of the hypoxia response element of the Vegf promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development.J Cell Physiol. 2006 Mar;206(3):749-58. doi: 10.1002/jcp.20525. J Cell Physiol. 2006. PMID: 16245301
-
Oxidative stress promotes ocular neovascularization.J Cell Physiol. 2009 Jun;219(3):544-52. doi: 10.1002/jcp.21698. J Cell Physiol. 2009. PMID: 19142872 Free PMC article.
-
Müller cell-derived VEGF is a significant contributor to retinal neovascularization.J Pathol. 2009 Dec;219(4):446-54. doi: 10.1002/path.2611. J Pathol. 2009. PMID: 19768732
-
[Role of VEGF in diseases of the retina].Arch Soc Esp Oftalmol. 2015 Mar;90 Suppl 1:3-5. doi: 10.1016/S0365-6691(15)30002-2. Arch Soc Esp Oftalmol. 2015. PMID: 25925044 Review. Spanish.
-
[Cell biology of intraocular vascular diseases].Nippon Ganka Gakkai Zasshi. 1999 Dec;103(12):923-47. Nippon Ganka Gakkai Zasshi. 1999. PMID: 10643294 Review. Japanese.
Cited by
-
A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease.Biochem Pharmacol. 2019 Jun;164:188-204. doi: 10.1016/j.bcp.2019.03.033. Epub 2019 Mar 21. Biochem Pharmacol. 2019. PMID: 30905657 Free PMC article. Review.
-
ADAM15 Is Functionally Associated with the Metastatic Progression of Human Bladder Cancer.PLoS One. 2016 Mar 1;11(3):e0150138. doi: 10.1371/journal.pone.0150138. eCollection 2016. PLoS One. 2016. PMID: 26930657 Free PMC article.
-
Identifying Novel Genes and Variants in Immune and Coagulation Pathways Associated with Macular Degeneration.Ophthalmol Sci. 2022 Aug 8;3(1):100206. doi: 10.1016/j.xops.2022.100206. eCollection 2023 Mar. Ophthalmol Sci. 2022. PMID: 36275200 Free PMC article.
-
Characterization of oxygen-induced retinopathy in mice carrying an inactivating point mutation in the catalytic site of ADAM15.Invest Ophthalmol Vis Sci. 2014 Sep 23;55(10):6774-82. doi: 10.1167/iovs.14-14472. Invest Ophthalmol Vis Sci. 2014. PMID: 25249606 Free PMC article.
-
Integrating predicted transcriptome from multiple tissues improves association detection.PLoS Genet. 2019 Jan 22;15(1):e1007889. doi: 10.1371/journal.pgen.1007889. eCollection 2019 Jan. PLoS Genet. 2019. PMID: 30668570 Free PMC article.
References
-
- Gould R J, Polokoff M A, Friedman P A, Huang T F, Holt J C, Cook J J, Niewiarowski S. Disintegrins: a family of integrin inhibitory proteins from viper venoms. Proc Soc Exp Biol Med. 1990;195:168–171. - PubMed
-
- Ari-Pekka J, Turner A J, Pelto-Huikko M, Karkkainen I, Ortiz R M. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. - PubMed
-
- Kratzschmar J, Lum L, Blobel C P. Metargidin, a membrane-anchored metalloprotease-disintegrin protein with an RGD integrin binding sequence. J Biol Chem. 1996;271:4593–4596. - PubMed
-
- Herren B, Raines E W, Ross R. Expression of a disintegrin-like protein in cultured human vascular cells and in vivo. FASEB J. 1997;11:173–180. - PubMed
-
- Zhang X-P, Kamata T, Yokoyama K, Puzon-McLaughlin W, Takada Y. Specific interaction of the recombinant disintegrin-like domain of MDC-15 (metargidin, ADAM-15) with integrin αvβ3. J Biol Chem. 1998;273:7345–7350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

