An Adam15 amplification loop promotes vascular endothelial growth factor-induced ocular neovascularization
- PMID: 18381816
- PMCID: PMC2493454
- DOI: 10.1096/fj.07-099283
An Adam15 amplification loop promotes vascular endothelial growth factor-induced ocular neovascularization
Abstract
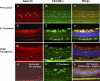
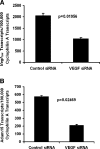
Proteins with a disintegrin and a metalloproteinase domain (ADAMs) are a family of membrane-bound proteinases that bind integrins through their disintegrin domain. In this study, we have found modest expression of ADAM15 in pericytes in normal retina and strong up-regulation of ADAM15 in retinal vascular endothelial cells in ischemic retina. Increased expression of vascular endothelial growth factor (VEGF) in the retina in the absence of ischemia also increased ADAM15 levels, and knockdown of Vegf mRNA in ischemic retina reduced Adam15 mRNA. Mice deficient in ADAM15 showed a significant reduction in ischemia-induced retinal neovascularization, choroidal neovascularization at rupture sites in Bruch's membrane, and VEGF-induced subretinal neovascularization. ADAM15-deficient mice also showed reduced levels of VEGF(164), VEGF receptor 1, and VEGF receptor 2 in ischemic retina. These data suggest that ADAM15 and VEGF participate in an amplification loop; VEGF increases expression of ADAM15, which in turn increases expression of VEGF and its receptors. Perturbation of the loop by elimination of ADAM15 suppresses ocular neovascularization in 3 different model systems, and thus ADAM15 provides a new therapeutic target for diseases complicated by neovascularization.
Figures
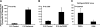




References
-
- Gould R J, Polokoff M A, Friedman P A, Huang T F, Holt J C, Cook J J, Niewiarowski S. Disintegrins: a family of integrin inhibitory proteins from viper venoms. Proc Soc Exp Biol Med. 1990;195:168–171. - PubMed
-
- Ari-Pekka J, Turner A J, Pelto-Huikko M, Karkkainen I, Ortiz R M. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. - PubMed
-
- Kratzschmar J, Lum L, Blobel C P. Metargidin, a membrane-anchored metalloprotease-disintegrin protein with an RGD integrin binding sequence. J Biol Chem. 1996;271:4593–4596. - PubMed
-
- Herren B, Raines E W, Ross R. Expression of a disintegrin-like protein in cultured human vascular cells and in vivo. FASEB J. 1997;11:173–180. - PubMed
-
- Zhang X-P, Kamata T, Yokoyama K, Puzon-McLaughlin W, Takada Y. Specific interaction of the recombinant disintegrin-like domain of MDC-15 (metargidin, ADAM-15) with integrin αvβ3. J Biol Chem. 1998;273:7345–7350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

