Water inertial reorientation: hydrogen bond strength and the angular potential
- PMID: 18381817
- PMCID: PMC2291089
- DOI: 10.1073/pnas.0801554105
Water inertial reorientation: hydrogen bond strength and the angular potential
Abstract
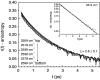
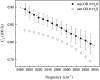
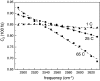
The short-time orientational relaxation of water is studied by ultrafast infrared pump-probe spectroscopy of the hydroxyl stretching mode (OD of dilute HOD in H(2)O). The anisotropy decay displays a sharp drop at very short times caused by inertial orientational motion, followed by a much slower decay that fully randomizes the orientation. Investigation of temperatures from 1 degrees C to 65 degrees C shows that the amplitude of the inertial component (extent of inertial angular displacement) depends strongly on the stretching frequency of the OD oscillator at higher temperatures, although the slow component is frequency-independent. The inertial component becomes frequency-independent at low temperatures. At high temperatures there is a correlation between the amplitude of the inertial decay and the strength of the O-D O hydrogen bond, but at low temperatures the correlation disappears, showing that a single hydrogen bond (OD O) is no longer a significant determinant of the inertial angular motion. It is suggested that the loss of correlation at lower temperatures is caused by the increased importance of collective effects of the extended hydrogen bonding network. By using a new harmonic cone model, the experimentally measured amplitudes of the inertial decays yield estimates of the characteristic frequencies of the intermolecular angular potential for various strengths of hydrogen bonds. The frequencies are in the range of approximately 400 cm(-1). A comparison with recent molecular dynamics simulations employing the simple point charge-extended water model at room temperature shows that the simulations qualitatively reflect the correlation between the inertial decay and the OD stretching frequency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Walrafen GE. Raman spectral studies of HDO in H2O. J Chem Phys. 1968;48:244–251.
-
- Frank HS, Wen W-Y. Structural aspects of ion-solvent interaction in aqueous solutions: A suggested picture of water structure. Discuss Faraday Soc. 1957;24:133–140.
-
- Pople JA. Molecular association in liquids II. A theory of the structure of water. Proc R Soc London Ser A. 1951;205:163–178.
-
- Bernal JD. The structure of liquids. Proc Roy Soc London Ser A. 1964;280:299–322.
-
- Falk M, Ford TA. Infrared spectrum and structure of liquid water. Can J Chem. 1966;44:1699.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

