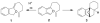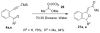Synthesis of alkylidenephthalans through fluoride-induced cyclization of electron-deficient 2-siloxymethylphenylacetylene derivatives
- PMID: 18382602
- PMCID: PMC1868474
- DOI: 10.1016/j.tet.2007.01.046
Synthesis of alkylidenephthalans through fluoride-induced cyclization of electron-deficient 2-siloxymethylphenylacetylene derivatives
Abstract
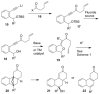
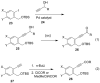
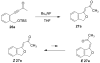
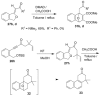
Fluoride-based deprotection of silylated 2-alkynylbenzyl alcohol derivatives featuring carbonyl-substituted alkynes results in the direct synthesis of alkylidenephthalan vinylogous esters. The reaction is selective for the Z alkylidenephthalans in a thermodynamically controlled process. Similar compounds are also produced in the coupling of Fischer carbene complexes with 2-alkynylbenzoyl derivatives in an aqueous solvent system. Subsequent acid-catalyzed inter- or intramolecular Diels-Alder reactions lead to hydronaphthalene or hydrophenanthrene derivatives.
Figures
References
-
-
For a mechanistic discussion, see: Friedrichsen W. Structural Chem. 1999;10:47–52. and references therein. For a review of isobenzofuran chemistry, see: Friedrichsen W. Adv Heterocycl Chem. 1999;73:1–96.
-
-
- Meegalla SK, Rodrigo R. J Org Chem. 1991;56:1882–1888.
-
- Meegalla SK, Rodrigo R. Synthesis. 1989:942–944.
-
- Bacchi A, Costa M, Della Ca N, Fabbricatore M, Fazio A, Gabriele B, Nasi C, Salerno G. Eur J Org Chem. 2004:574–585.
-
- Jiang D, Herndon JW. Org Lett. 2000;2:1267–1269. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources