Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing
- PMID: 18382669
- PMCID: PMC2270908
- DOI: 10.1371/journal.pone.0001886
Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing
Erratum in
-
Correction: Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing.PLoS One. 2024 Apr 15;19(4):e0302417. doi: 10.1371/journal.pone.0302417. eCollection 2024. PLoS One. 2024. PMID: 38620023 Free PMC article.
Abstract
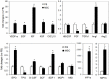
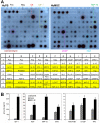
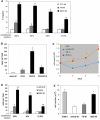
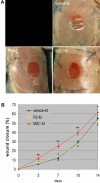
Bone marrow derived mesenchymal stem cells (BM-MSCs) have been shown to enhance wound healing; however, the mechanisms involved are barely understood. In this study, we examined paracrine factors released by BM-MSCs and their effects on the cells participating in wound healing compared to those released by dermal fibroblasts. Analyses of BM-MSCs with Real-Time PCR and of BM-MSC-conditioned medium by antibody-based protein array and ELISA indicated that BM-MSCs secreted distinctively different cytokines and chemokines, such as greater amounts of VEGF-alpha, IGF-1, EGF, keratinocyte growth factor, angiopoietin-1, stromal derived factor-1, macrophage inflammatory protein-1alpha and beta and erythropoietin, compared to dermal fibroblasts. These molecules are known to be important in normal wound healing. BM-MSC-conditioned medium significantly enhanced migration of macrophages, keratinocytes and endothelial cells and proliferation of keratinocytes and endothelial cells compared to fibroblast-conditioned medium. Moreover, in a mouse model of excisional wound healing, where concentrated BM-MSC-conditioned medium was applied, accelerated wound healing occurred compared to administration of pre-conditioned or fibroblast-conditioned medium. Analysis of cell suspensions derived from the wound by FACS showed that wounds treated with BM-MSC-conditioned medium had increased proportions of CD4/80-positive macrophages and Flk-1-, CD34- or c-kit-positive endothelial (progenitor) cells compared to wounds treated with pre-conditioned medium or fibroblast-conditioned medium. Consistent with the above findings, immunohistochemical analysis of wound sections showed that wounds treated with BM-MSC-conditioned medium had increased abundance of macrophages. Our results suggest that factors released by BM-MSCs recruit macrophages and endothelial lineage cells into the wound thus enhancing wound healing.
Conflict of interest statement
Figures
References
-
- Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
-
- Singer AJ, Clark RA. Cutaneous wound healing. N.Engl.J Med. 1999;341:738–746. - PubMed
-
- Falanga V. Wound healing and its impairment in the diabetic foot. The Lancet. 2005;366:1736–1743. - PubMed
-
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. - PubMed
-
- Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

