Different effect of proteasome inhibition on vesicular stomatitis virus and poliovirus replication
- PMID: 18382670
- PMCID: PMC2268745
- DOI: 10.1371/journal.pone.0001887
Different effect of proteasome inhibition on vesicular stomatitis virus and poliovirus replication
Abstract
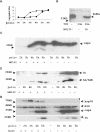
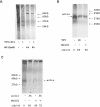
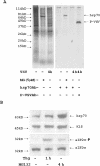
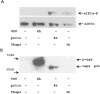
Proteasome activity is an important part of viral replication. In this study, we examined the effect of proteasome inhibitors on the replication of vesicular stomatitis virus (VSV) and poliovirus. We found that the proteasome inhibitors significantly suppressed VSV protein synthesis, virus accumulation, and protected infected cells from toxic effect of VSV replication. In contrast, poliovirus replication was delayed, but not diminished in the presence of the proteasome inhibitors MG132 and Bortezomib. We also found that inhibition of proteasomes stimulated stress-related processes, such as accumulation of chaperone hsp70, phosphorylation of eIF2alpha, and overall inhibition of translation. VSV replication was sensitive to this stress with significant decline in replication process. Poliovirus growth was less sensitive with only delay in replication. Inhibition of proteasome activity suppressed cellular and VSV protein synthesis, but did not reduce poliovirus protein synthesis. Protein kinase GCN2 supported the ability of proteasome inhibitors to attenuate general translation and to suppress VSV replication. We propose that different mechanisms of translational initiation by VSV and poliovirus determine their sensitivity to stress induced by the inhibition of proteasomes. To our knowledge, this is the first study that connects the effect of stress induced by proteasome inhibition with the efficiency of viral infection.
Conflict of interest statement
Figures







Similar articles
-
Investigation of the eIF2alpha phosphorylation mechanism in response to proteasome inhibition in melanoma and breast cancer cells.Mol Biol (Mosk). 2010 Sep-Oct;44(5):859-66. Mol Biol (Mosk). 2010. PMID: 21090173
-
The ubiquitin-proteasome system plays an important role during various stages of the coronavirus infection cycle.J Virol. 2010 Aug;84(15):7869-79. doi: 10.1128/JVI.00485-10. Epub 2010 May 19. J Virol. 2010. PMID: 20484504 Free PMC article.
-
Proteasome inhibition in vivo promotes survival in a lethal murine model of severe acute respiratory syndrome.J Virol. 2010 Dec;84(23):12419-28. doi: 10.1128/JVI.01219-10. Epub 2010 Sep 22. J Virol. 2010. PMID: 20861244 Free PMC article.
-
The ubiquitin-proteasome system (UPS) and the mechanism of action of bortezomib.Curr Pharm Des. 2011;17(15):1483-99. doi: 10.2174/138161211796197124. Curr Pharm Des. 2011. PMID: 21504411 Review.
-
Proteasome inhibitors in the clinical setting: benefits and strategies to overcome multiple myeloma resistance to proteasome inhibitors.Drugs R D. 2007;8(1):1-12. doi: 10.2165/00126839-200708010-00001. Drugs R D. 2007. PMID: 17249845 Review.
Cited by
-
Human metapneumovirus inhibits IFN-β signaling by downregulating Jak1 and Tyk2 cellular levels.PLoS One. 2011;6(9):e24496. doi: 10.1371/journal.pone.0024496. Epub 2011 Sep 19. PLoS One. 2011. PMID: 21949722 Free PMC article.
-
Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses.PLoS Pathog. 2013 Mar;9(3):e1003231. doi: 10.1371/journal.ppat.1003231. Epub 2013 Mar 21. PLoS Pathog. 2013. PMID: 23555247 Free PMC article.
-
Targeting Nup358/RanBP2 by a viral protein disrupts stress granule formation.PLoS Pathog. 2022 Dec 1;18(12):e1010598. doi: 10.1371/journal.ppat.1010598. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36455064 Free PMC article.
-
Proteasomal Protein Degradation: Adaptation of Cellular Proteolysis With Impact on Virus-and Cytokine-Mediated Damage of Heart Tissue During Myocarditis.Front Immunol. 2018 Nov 28;9:2620. doi: 10.3389/fimmu.2018.02620. eCollection 2018. Front Immunol. 2018. PMID: 30546359 Free PMC article. Review.
-
The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling.PLoS Pathog. 2011 Mar;7(3):e1001311. doi: 10.1371/journal.ppat.1001311. Epub 2011 Mar 10. PLoS Pathog. 2011. PMID: 21436888 Free PMC article.
References
-
- Weissman AM. Themes and variations on ubiquitination. Nat Rev Mol Cell Biol. 2001;2:169–178. - PubMed
-
- Kloetzel PM. Antigen processing by the proteasome. Nat Rev Mol Cell Biol. 2001;2:179–187. - PubMed
-
- Roos-Mattjus P, Sistonen L. The ubiquitin-proteasome pathway. Ann Med. 2004;36:285–295. - PubMed
-
- Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. - PubMed
-
- Caraglia M, Marra M, Pelaia G, Maselli R, Caputi M, et al. Alpha-interferon and its effects on signal transduction pathways. J Cell Physiol. 2005;202:323–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

