A comparison of donor-acceptor pairs for genetically encoded FRET sensors: application to the Epac cAMP sensor as an example
- PMID: 18382687
- PMCID: PMC2271053
- DOI: 10.1371/journal.pone.0001916
A comparison of donor-acceptor pairs for genetically encoded FRET sensors: application to the Epac cAMP sensor as an example
Abstract
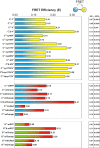
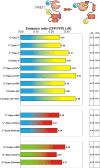
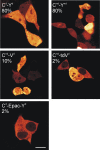
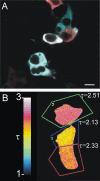
We recently reported on CFP-Epac-YFP, an Epac-based single polypeptide FRET reporter to resolve cAMP levels in living cells. In this study, we compared and optimized the fluorescent protein donor/acceptor pairs for use in biosensors such as CFP-Epac-YFP. Our strategy was to prepare a wide range of constructs consisting of different donor and acceptor fluorescent proteins separated by a short linker. Constructs were expressed in HEK293 cells and tested for FRET and other relevant properties. The most promising pairs were subsequently used in an attempt to improve the FRET span of the Epac-based cAMP sensor. The results show significant albeit not perfect correlation between performance in the spacer construct and in the Epac sensor. Finally, this strategy enabled us to identify improved sensors both for detection by sensitized emission and by fluorescent lifetime imaging. The present overview should be helpful in guiding development of future FRET sensors.
Conflict of interest statement
Figures



Similar articles
-
Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator.EMBO Rep. 2004 Dec;5(12):1176-80. doi: 10.1038/sj.embor.7400290. EMBO Rep. 2004. PMID: 15550931 Free PMC article.
-
Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity.PLoS One. 2015 Apr 14;10(4):e0122513. doi: 10.1371/journal.pone.0122513. eCollection 2015. PLoS One. 2015. PMID: 25875503 Free PMC article.
-
A mTurquoise-based cAMP sensor for both FLIM and ratiometric read-out has improved dynamic range.PLoS One. 2011 Apr 29;6(4):e19170. doi: 10.1371/journal.pone.0019170. PLoS One. 2011. PMID: 21559477 Free PMC article.
-
Interrogating cyclic AMP signaling using optical approaches.Cell Calcium. 2017 Jun;64:47-56. doi: 10.1016/j.ceca.2017.02.010. Epub 2017 Mar 1. Cell Calcium. 2017. PMID: 28274483 Free PMC article. Review.
-
Recent Advances in Development of Genetically Encoded Fluorescent Sensors.Methods Enzymol. 2017;589:1-49. doi: 10.1016/bs.mie.2017.01.019. Epub 2017 Mar 9. Methods Enzymol. 2017. PMID: 28336060 Review.
Cited by
-
Human and viral Golgi anti-apoptotic proteins (GAAPs) oligomerize via different mechanisms and monomeric GAAP inhibits apoptosis and modulates calcium.J Biol Chem. 2013 May 3;288(18):13057-67. doi: 10.1074/jbc.M112.414367. Epub 2013 Mar 18. J Biol Chem. 2013. PMID: 23508950 Free PMC article.
-
Astrocyte VAMP3 vesicles undergo Ca2+ -independent cycling and modulate glutamate transporter trafficking.J Physiol. 2015 Jul 1;593(13):2807-32. doi: 10.1113/JP270362. Epub 2015 May 18. J Physiol. 2015. PMID: 25864578 Free PMC article.
-
In vivo characterisation of fluorescent proteins in budding yeast.Sci Rep. 2019 Feb 19;9(1):2234. doi: 10.1038/s41598-019-38913-z. Sci Rep. 2019. PMID: 30783202 Free PMC article.
-
Intracellular cAMP Sensor EPAC: Physiology, Pathophysiology, and Therapeutics Development.Physiol Rev. 2018 Apr 1;98(2):919-1053. doi: 10.1152/physrev.00025.2017. Physiol Rev. 2018. PMID: 29537337 Free PMC article. Review.
-
Multiplexed 3D FRET imaging in deep tissue of live embryos.Sci Rep. 2015 Sep 21;5:13991. doi: 10.1038/srep13991. Sci Rep. 2015. PMID: 26387920 Free PMC article.
References
-
- Förster T. Zwischenmolekulare Energie Wanderungen und Fluoreszenz. Annals of Physics. 1948;2:57–75.
-
- Patterson GH, Piston DW, Barisas BG. Forster distances between green fluorescent protein pairs. Anal Biochem. 2000;284:438–440. - PubMed
-
- Jares-Erijman EA, Jovin TM. FRET imaging. Nat Biotechnol. 2003;21:1387–1395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

