Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE
- PMID: 18382691
- PMCID: PMC2271128
- DOI: 10.1371/journal.pone.0001924
Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE
Abstract
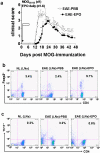
Background: Beneficial effects of short-term erythropoietin (EPO) therapy have been demonstrated in several animal models of acute neurologic injury, including experimental autoimmune encephalomyelitis (EAE)--the animal model of multiple sclerosis. We have found that EPO treatment substantially reduces the acute clinical paralysis seen in EAE mice and this improvement is accompanied by a large reduction in the mononuclear cell infiltration and downregulation of glial MHC class II expression within the inflamed CNS. Other reports have recently indicated that peripherally generated anti-inflammatory CD4(+)Foxp3(+) regulatory T cells (Tregs) and the IL17-producing CD4+ T helper cell (Th17) subpopulations play key antagonistic roles in EAE pathogenesis. However, no information regarding the effects of EPO therapy on the behavior of the general mononuclear-lymphocyte population, Tregs or Th17 cells in EAE has emerged.
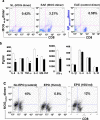
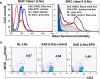
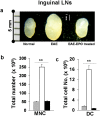
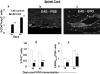
Methods and findings: We first determined in vivo that EPO therapy markedly suppressed MOG specific T cell proliferation and sharply reduced the number of reactive dendritic cells (CD11c positive) in EAE lymph nodes during both inductive and later symptomatic phases of MOG(35-55) induced EAE. We then determined the effect in vivo of EPO on numbers of peripheral Treg cells and Th17 cells. We found that EPO treatment modulated immune balance in both the periphery and the inflamed spinal cord by promoting a large expansion in Treg cells, inhibiting Th17 polarization and abrogating proliferation of the antigen presenting dendritic cell population. Finally we utilized tissue culture assays to show that exposure to EPO in vitro similarly downregulated MOG-specific T cell proliferation and also greatly suppressed T cell production of pro-inflammatory cytokines.
Conclusions: Taken together, our findings reveal an important new locus whereby EPO induces substantial long-term tissue protection in the host through signaling to several critical subsets of immune cells that reside in the peripheral lymphatic system.
Conflict of interest statement
Figures
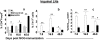
Similar articles
-
Erythropoietin enhances endogenous haem oxygenase-1 and represses immune responses to ameliorate experimental autoimmune encephalomyelitis.Clin Exp Immunol. 2010 Nov;162(2):210-23. doi: 10.1111/j.1365-2249.2010.04238.x. Clin Exp Immunol. 2010. PMID: 21069936 Free PMC article.
-
Erythropoietin reduces experimental autoimmune encephalomyelitis severity via neuroprotective mechanisms.J Neuroinflammation. 2017 Oct 13;14(1):202. doi: 10.1186/s12974-017-0976-5. J Neuroinflammation. 2017. PMID: 29029628 Free PMC article.
-
Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance.Brain Behav Immun. 2015 Nov;50:101-114. doi: 10.1016/j.bbi.2015.06.021. Epub 2015 Jun 27. Brain Behav Immun. 2015. PMID: 26130320
-
A Distinct Region in Erythropoietin that Induces Immuno/Inflammatory Modulation and Tissue Protection.Neurotherapeutics. 2015 Oct;12(4):850-61. doi: 10.1007/s13311-015-0379-1. Neurotherapeutics. 2015. PMID: 26271954 Free PMC article. Review.
-
Green tea EGCG, T cells, and T cell-mediated autoimmune diseases.Mol Aspects Med. 2012 Feb;33(1):107-18. doi: 10.1016/j.mam.2011.10.001. Epub 2011 Oct 14. Mol Aspects Med. 2012. PMID: 22020144 Review.
Cited by
-
Erythropoietin enhances immunostimulatory properties of immature dendritic cells.Clin Exp Immunol. 2011 Aug;165(2):202-10. doi: 10.1111/j.1365-2249.2011.04417.x. Epub 2011 Jun 7. Clin Exp Immunol. 2011. PMID: 21649646 Free PMC article.
-
Enhancing the ability of experimental autoimmune encephalomyelitis to serve as a more rigorous model of multiple sclerosis through refinement of the experimental design.Comp Med. 2009 Apr;59(2):112-28. Comp Med. 2009. PMID: 19389303 Free PMC article.
-
An Overview of Peptide-Based Molecules as Potential Drug Candidates for Multiple Sclerosis.Molecules. 2021 Aug 28;26(17):5227. doi: 10.3390/molecules26175227. Molecules. 2021. PMID: 34500662 Free PMC article. Review.
-
Current status of the immunomodulation and immunomediated therapeutic strategies for multiple sclerosis.Clin Dev Immunol. 2012;2012:970789. doi: 10.1155/2012/970789. Epub 2011 Dec 6. Clin Dev Immunol. 2012. PMID: 22203863 Free PMC article. Review.
-
Animal models of multiple sclerosis--potentials and limitations.Prog Neurobiol. 2010 Nov;92(3):386-404. doi: 10.1016/j.pneurobio.2010.06.005. Epub 2010 Jun 15. Prog Neurobiol. 2010. PMID: 20558237 Free PMC article. Review.
References
-
- Sebzda E, Mariathasan S, Ohteki T, Jones R, Bachmann MF, et al. Selection of the T cell repertoire. Annu Rev Immunol. 1999;17:829–874. - PubMed
-
- Baldwin KK, Trenchak BP, Altman JD, Davis MM. Negative selection of T cells occurs throughout thymic development. J Immunol. 1999;163:689–698. - PubMed
-
- Stockinger B. T lymphocyte tolerance: from thymic deletion to peripheral control mechanisms. Adv Immunol. 1999;71:229–265. - PubMed
-
- Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

