Kinetoplastids: related protozoan pathogens, different diseases
- PMID: 18382742
- PMCID: PMC2276762
- DOI: 10.1172/JCI33945
Kinetoplastids: related protozoan pathogens, different diseases
Abstract
Kinetoplastids are a group of flagellated protozoans that include the species Trypanosoma and Leishmania, which are human pathogens with devastating health and economic effects. The sequencing of the genomes of some of these species has highlighted their genetic relatedness and underlined differences in the diseases that they cause. As we discuss in this Review, steady progress using a combination of molecular, genetic, immunologic, and clinical approaches has substantially increased understanding of these pathogens and important aspects of the diseases that they cause. Consequently, the paths for developing additional measures to control these "neglected diseases" are becoming increasingly clear, and we believe that the opportunities for developing the drugs, diagnostics, vaccines, and other tools necessary to expand the armamentarium to combat these diseases have never been better.
Figures
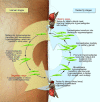
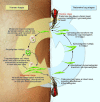

References
-
- Burri, C., and Brun, R. 2003. Human African trypanosomiasis. InManson’s tropical diseases. G.C. Cook and A.I. Zumla, editors. 21st edition. W.B. Saunders/Elsevier. Edinburgh, United Kingdom. 1303–1323.
-
- [No authors listed]. . Human African trypanosomiasis (sleeping sickness): epidemiological update. Wkly. Epidemiol. Rec. 2006;81:71–80. - PubMed
-
- Radwanska M., et al. The serum resistance-associated gene as a diagnostic tool for the detection of Trypanosoma brucei rhodesiense. Am. J. Trop. Med. Hyg. 2002;67:684–690. - PubMed
-
- Radwanska M., et al. Novel primer sequences for polymerase chain reaction-based detection of Trypanosoma brucei gambiense. Am. J. Trop. Med. Hyg. 2002;67:289–295. - PubMed
-
- Magnus E., Vervoort T., Van Meirvenne N. A card-agglutination test with stained trypanosomes (C.A.T.T.) for the serological diagnosis of T. B. gambiense trypanosomiasis. Ann. Soc. Belg. Med. Trop. 1978;58:169–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

