Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice
- PMID: 18382763
- PMCID: PMC2276399
- DOI: 10.1172/JCI34257
Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice
Abstract
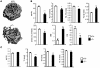
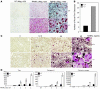
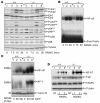
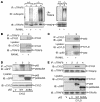
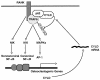
Osteoclastogenesis is a tightly regulated biological process, and deregulation can lead to severe bone disorders such as osteoporosis. The regulation of osteoclastic signaling is incompletely understood, but ubiquitination of TNF receptor-associated factor 6 (TRAF6) has recently been shown to be important in mediating this process. We therefore investigated the role of the recently identified deubiquitinating enzyme CYLD in osteoclastogenesis and found that mice with a genetic deficiency of CYLD had aberrant osteoclast differentiation and developed severe osteoporosis. Cultured osteoclast precursors derived from CYLD-deficient mice were hyperresponsive to RANKL-induced differentiation and produced more and larger osteoclasts than did controls upon stimulation. We assessed the expression pattern of CYLD and found that it was drastically upregulated during RANKL-induced differentiation of preosteoclasts. Furthermore, CYLD negatively regulated RANK signaling by inhibiting TRAF6 ubiquitination and activation of downstream signaling events. Interestingly, we found that CYLD interacted physically with the signaling adaptor p62 and thereby was recruited to TRAF6. These findings establish CYLD as a crucial negative regulator of osteoclastogenesis and suggest its involvement in the p62/TRAF6 signaling axis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

