The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice
- PMID: 18382764
- PMCID: PMC2276397
- DOI: 10.1172/JCI33342
The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice
Abstract
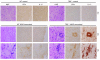
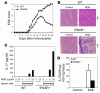
IFN-beta, a type I IFN, is widely used for the treatment of MS. However, the mechanisms behind its therapeutic efficacy are not well understood. Using a murine model of MS, EAE, we demonstrate that the Th17-mediated development of autoimmune disease is constrained by Toll-IL-1 receptor domain-containing adaptor inducing IFN-beta-dependent (TRIF-dependent) type I IFN production and its downstream signaling pathway. Mice with defects in TRIF or type I IFN receptor (IFNAR) developed more severe EAE. Notably, these mice exhibited marked CNS inflammation, as manifested by increased IL-17 production. In addition, IFNAR-dependent signaling events were essential for negatively regulating Th17 development. Finally, IFN-beta-mediated IL-27 production by innate immune cells was critical for the immunoregulatory role of IFN-beta in the CNS autoimmune disease. Together, our findings not only may provide a molecular mechanism for the clinical benefits of IFN-beta in MS but also demonstrate a regulatory role for type I IFN induction and its downstream signaling pathways in limiting Th17 development and autoimmune inflammation.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

