An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension
- PMID: 18382765
- PMCID: PMC2276393
- DOI: 10.1172/JCI32503
An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension
Abstract
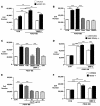
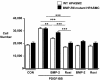
Loss-of-function mutations in bone morphogenetic protein receptor II (BMP-RII) are linked to pulmonary arterial hypertension (PAH); the ligand for BMP-RII, BMP-2, is a negative regulator of SMC growth. Here, we report an interplay between PPARgamma and its transcriptional target apoE downstream of BMP-2 signaling. BMP-2/BMP-RII signaling prevented PDGF-BB-induced proliferation of human and murine pulmonary artery SMCs (PASMCs) by decreasing nuclear phospho-ERK and inducing DNA binding of PPARgamma that is independent of Smad1/5/8 phosphorylation. Both BMP-2 and a PPARgamma agonist stimulated production and secretion of apoE by SMCs. Using a variety of methods, including short hairpin RNAi in human PASMCs, PAH patient-derived BMP-RII mutant PASMCs, a PPARgamma antagonist, and PASMCs isolated from PPARgamma- and apoE-deficient mice, we demonstrated that the antiproliferative effect of BMP-2 was BMP-RII, PPARgamma, and apoE dependent. Furthermore, we created mice with targeted deletion of PPARgamma in SMCs and showed that they spontaneously developed PAH, as indicated by elevated RV systolic pressure, RV hypertrophy, and increased muscularization of the distal pulmonary arteries. Thus, PPARgamma-mediated events could protect against PAH, and PPARgamma agonists may reverse PAH in patients with or without BMP-RII dysfunction.
Figures

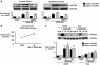




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

