Characterization of nonderivatized plant cell walls using high-resolution solution-state NMR spectroscopy
- PMID: 18383438
- PMCID: PMC5826555
- DOI: 10.1002/mrc.2201
Characterization of nonderivatized plant cell walls using high-resolution solution-state NMR spectroscopy
Erratum in
- Magn Reson Chem. 2008 Sep;46(9). doi: 10.1002/mrc.2251 doi: 10.1002/mrc.2251
Abstract
A recently described plant cell wall dissolution system has been modified to use perdeuterated solvents to allow direct in-NMR-tube dissolution and high-resolution solution-state NMR of the whole cell wall without derivatization. Finely ground cell wall material dissolves in a solvent system containing dimethylsulfoxide-d(6) and 1-methylimidazole-d(6) in a ratio of 4:1 (v/v), keeping wood component structures mainly intact in their near-native state. Two-dimensional NMR experiments, using gradient-HSQC (heteronuclear single quantum coherence) 1-bond (13)C--(1)H correlation spectroscopy, on nonderivatized cell wall material from a representative gymnosperm pinus taeda (loblolly pine), an angiosperm Populus tremuloides (quaking aspen), and a herbaceous plant Hibiscus cannabinus (kenaf) demonstrate the efficacy of the system. We describe a method to synthesize 1-methylimidazole-d(6) with a high degree of perdeuteration, thus allowing cell wall dissolution and NMR characterization of nonderivatized plant cell wall structures.
Copyright (c) 2008 John Wiley & Sons, Ltd
Figures




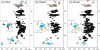


References
-
- Barron PF, Frost RL, Doimo L, Kennedy MJ. J. Macromol. Sci.-Chem. 1985;A22:303.
-
- Sterk H, Sattler W, Esterbauer H. Carbohydr. Res. 1987;164:85.
-
- Bardet M, Emsley L, Vincendon M. Solid State Nucl. Magn. Reson. 1997;8:25. - PubMed
-
- Johnson CE, Smernik RJ, Siccama TG, Kiemle DK, Xu Z, Vogt DJ. Can. J. For. Res. 2005;35:1821.
-
- Lu F, Ralph J. Plant J. 2003;35:535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

