Using imaging and genetics in zebrafish to study developing spinal circuits in vivo
- PMID: 18383546
- PMCID: PMC3579555
- DOI: 10.1002/dneu.20617
Using imaging and genetics in zebrafish to study developing spinal circuits in vivo
Abstract
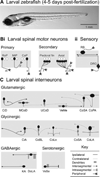
Imaging and molecular approaches are perfectly suited to young, transparent zebrafish (Danio rerio), where they have allowed novel functional studies of neural circuits and their links to behavior. Here, we review cutting-edge optical and genetic techniques used to dissect neural circuits in vivo and discuss their application to future studies of developing spinal circuits using living zebrafish. We anticipate that these experiments will reveal general principles governing the assembly of neural circuits that control movements.
Figures





Similar articles
-
Modeling spinal locomotor circuits for movements in developing zebrafish.Elife. 2021 Sep 2;10:e67453. doi: 10.7554/eLife.67453. Elife. 2021. PMID: 34473059 Free PMC article.
-
Imaging circuit formation in zebrafish.Dev Neurobiol. 2012 Mar;72(3):346-57. doi: 10.1002/dneu.20874. Dev Neurobiol. 2012. PMID: 21309080 Review.
-
Spinal interneurons differentiate sequentially from those driving the fastest swimming movements in larval zebrafish to those driving the slowest ones.J Neurosci. 2009 Oct 28;29(43):13566-77. doi: 10.1523/JNEUROSCI.3277-09.2009. J Neurosci. 2009. PMID: 19864569 Free PMC article.
-
Movement, technology and discovery in the zebrafish.Curr Opin Neurobiol. 2011 Feb;21(1):110-5. doi: 10.1016/j.conb.2010.09.011. Epub 2010 Oct 20. Curr Opin Neurobiol. 2011. PMID: 20970321 Free PMC article. Review.
-
Live-imaging of astrocyte morphogenesis and function in zebrafish neural circuits.Nat Neurosci. 2020 Oct;23(10):1297-1306. doi: 10.1038/s41593-020-0703-x. Epub 2020 Sep 7. Nat Neurosci. 2020. PMID: 32895565 Free PMC article.
Cited by
-
Integrating anatomy and function for zebrafish circuit analysis.Front Neural Circuits. 2013 Apr 23;7:74. doi: 10.3389/fncir.2013.00074. eCollection 2013. Front Neural Circuits. 2013. PMID: 23630469 Free PMC article. Review.
-
Optogenetic Dissection of Neuronal Circuits in Zebrafish using Viral Gene Transfer and the Tet System.Front Neural Circuits. 2009 Dec 11;3:21. doi: 10.3389/neuro.04.021.2009. eCollection 2009. Front Neural Circuits. 2009. PMID: 20126518 Free PMC article.
-
Spinal Hb9::Cre-derived excitatory interneurons contribute to rhythm generation in the mouse.Sci Rep. 2017 Jan 27;7:41369. doi: 10.1038/srep41369. Sci Rep. 2017. PMID: 28128321 Free PMC article.
-
Glutamate drives the touch response through a rostral loop in the spinal cord of zebrafish embryos.Dev Neurobiol. 2009 Oct;69(12):780-95. doi: 10.1002/dneu.20741. Dev Neurobiol. 2009. PMID: 19634126 Free PMC article.
-
Some principles of organization of spinal neurons underlying locomotion in zebrafish and their implications.Ann N Y Acad Sci. 2010 Jun;1198:94-104. doi: 10.1111/j.1749-6632.2010.05539.x. Ann N Y Acad Sci. 2010. PMID: 20536924 Free PMC article.
References
-
- Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. - PubMed
-
- Aramaki S, Hatta K. Visualizing neurons one-by-one in vivo: Optical dissection and reconstruction of neural networks with reversible fluorescent proteins. Dev Dyn. 2006;235:2192–2199. - PubMed
-
- Bargmann CI, Hartwieg E, Horvitz HR. Odorantselective genes and neurons mediate olfaction in Celegans. Cell. 1993;74:515–527. - PubMed
-
- Bartelmez GW. Mauthner’s cell and the nucleus motorius tegmenti. J Comp Neurol. 1915;25:87–128.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

