Inhibition of HBV gene expression and replication by stably expressed interferon-alpha1 via adeno-associated viral vectors
- PMID: 18383553
- PMCID: PMC7166674
- DOI: 10.1002/jgm.1174
Inhibition of HBV gene expression and replication by stably expressed interferon-alpha1 via adeno-associated viral vectors
Abstract
Background: Interferon-alpha2 (IFNalpha2) is routinely used for anti-hepatitis B virus (HBV) treatment. However, the therapeutic efficiency is unsatisfactory, particularly in East Asia. Such inefficiency might be a result of the short half-life, relatively low local concentration and strong side-effects of interferons. Frequent and repeated injection is also a big burden for patients. In the present study, a single dose of vector-delivered IFNalpha1 was tested for its anti-HBV effects.
Methods: Adeno-associated viral vector (AAV-IFNalpha1) was generated to deliver the IFNalpha1 gene into hepatocytes. IFNalpha1, hepatitis B surface (HBsAg) and e (HBeAg) antigens were measured by enzyme-linked immunosorbent assay and/or western blotting. The level of viral DNA was measured by quantitative real-time polymerase chain reaction.
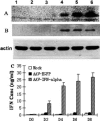
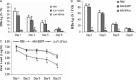
Results: AAV-IFNalpha1 effectively transduced HBV-producing cells (HepAD38) and mouse hepatocytes, where IFNalpha1 was expressed in a stable manner. Both intracellular and extracellular HBsAg and HBeAg were significantly reduced in vitro. In the HBV-producing mice, the concentration of IFNalpha1 in the liver was eight-fold higher than that in plasma. Compared with control groups, HBeAg/HBsAg antigen levels were reduced by more than ten-fold from day 1-5, and dropped to an undetectable level on day 9 in the AAV-IFNalpha1 group. Concurrently, the level of viral DNA decreased over 30-fold for several weeks.
Conclusions: A single dose administration of AAV-IFNalpha1 viral vector displayed prolonged transgene expression and superior antiviral effects both in vitro and in vivo. Therefore, the use of AAV-IFNalpha1 might be a potential alternative strategy for anti-HBV therapy.
Figures




Similar articles
-
Inhibition of HBV replication and gene expression in vitro and in vivo with a single AAV vector delivering two shRNA molecules.BMB Rep. 2009 Jan 31;42(1):59-64. doi: 10.5483/bmbrep.2009.42.1.059. BMB Rep. 2009. PMID: 19192395
-
A new HDV mouse model identifies mitochondrial antiviral signaling protein (MAVS) as a key player in IFN-β induction.J Hepatol. 2017 Oct;67(4):669-679. doi: 10.1016/j.jhep.2017.05.010. Epub 2017 May 18. J Hepatol. 2017. PMID: 28527664
-
The anti-HBV effect mediated by a novel recombinant eukaryotic expression vector for IFN-α.Virol J. 2013 Aug 29;10:270. doi: 10.1186/1743-422X-10-270. Virol J. 2013. PMID: 23984795 Free PMC article.
-
New options in the treatment of chronic hepatitis.Adv Exp Med Biol. 2003;531:191-8. doi: 10.1007/978-1-4615-0059-9_15. Adv Exp Med Biol. 2003. PMID: 12916791 Review.
-
Advances in designing Adeno-associated viral vectors for development of anti-HBV gene therapeutics.Virol J. 2021 Dec 13;18(1):247. doi: 10.1186/s12985-021-01715-9. Virol J. 2021. PMID: 34903258 Free PMC article. Review.
References
-
- Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet 2003; 362: 2089–2094. - PubMed
-
- Ocama P, Opio CK, Lee WM. Hepatitis B virus infection: current status. Am J Med 2005; 118: 1413. - PubMed
-
- Tang ZY, Yu YQ, Zhou XD, et al. Progress and prospects in hepatocellular carcinoma surgery. Ann Chir 1998; 52: 558–563. - PubMed
-
- Arauz‐Ruiz P, Norder H, Robertson BH, et al. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J Gen Virol 2002; 83: 2059–2073. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

