Interfaces in graded coatings on titanium-based implants
- PMID: 18384170
- PMCID: PMC3755615
- DOI: 10.1002/jbm.a.31935
Interfaces in graded coatings on titanium-based implants
Abstract
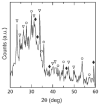
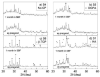
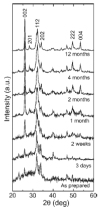
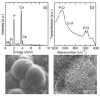
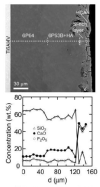
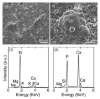
Graded bilayered glass-ceramic composite coatings on Ti6Al4V substrates were fabricated using an enameling technique. The layers consisted of a mixture of glasses in the CaO-MgO-Na(2)O-K(2)O-P(2)O(5) system with different amounts of calcium phosphates (CPs). Optimum firing conditions have been determined for the fabrication of coatings having good adhesion to the metal, while avoiding deleterious reactions between the glass and the ceramic particles. The final coatings do not crack or delaminate. The use of high-silica layers (>60 wt % SiO(2)) in contact with the alloy promotes long-term stability of the coating; glass-metal adhesion is achieved through the formation of a nanostructured Ti(5)Si(3) layer. A surface layer containing a mixture of a low-silica glass ( approximately 53 wt % SiO(2)) and synthetic hydroxyapatite particles promotes the precipitation of new apatite during tests in vitro. The in vitro behavior of the coatings in simulated body fluid depends both on the composition of the glass matrix and the CP particles, and is strongly affected by the coating design and the firing conditions.
Figures








References
-
- Hench LL. The story of bioglass. J Mater Sci Mater Med. 2006;17:967–978. Links. - PubMed
-
- Ma J, Chen CZ, Yao L, Bao QH. Characterization of some methods of preparation for bioactive glass coating on implants. Surf Rev Lett. 2006;13:93–102. Links.
-
- Zhao YF, Chen CZ, Wang DG. The current techniques for preparing bioglass coatings. Surf Rev Lett. 2005;12:505–513. Links.
-
- Yilmaz S, Ipek M, Celebi GF, Bindal C. The effect of bond coat on mechanical properties of plasma-sprayed Al2O3 and Al2O3-13 wt% TiO2 coatings on AISI 316L stainless steel. Vacuum. 2005;77:315–321. Links.
-
- Li P, deGroot K, Kokubo T. Bioactive Ca-10(PO4)(6)(OH)2-TiO2 composite coating prepared by sol-gel process. J Sol-Gel Sci Technol. 1996;7:27–34. Links.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

