Chromosomal gene movements reflect the recent origin and biology of therian sex chromosomes
- PMID: 18384235
- PMCID: PMC2276528
- DOI: 10.1371/journal.pbio.0060080
Chromosomal gene movements reflect the recent origin and biology of therian sex chromosomes
Abstract
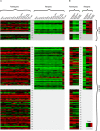
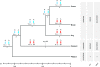
Mammalian sex chromosomes stem from ancestral autosomes and have substantially differentiated. It was shown that X-linked genes have generated duplicate intronless gene copies (retrogenes) on autosomes due to this differentiation. However, the precise driving forces for this out-of-X gene "movement" and its evolutionary onset are not known. Based on expression analyses of male germ-cell populations, we here substantiate and extend the hypothesis that autosomal retrogenes functionally compensate for the silencing of their X-linked housekeeping parental genes during, but also after, male meiotic sex chromosome inactivation (MSCI). Thus, sexually antagonistic forces have not played a major role for the selective fixation of X-derived gene copies in mammals. Our dating analyses reveal that although retrogenes were produced ever since the common mammalian ancestor, selectively driven retrogene export from the X only started later, on the placental mammal (eutherian) and marsupial (metatherian) lineages, respectively. Together, these observations suggest that chromosome-wide MSCI emerged close to the eutherian-marsupial split approximately 180 million years ago. Given that MSCI probably reflects the spread of the recombination barrier between the X and Y, crucial for their differentiation, our data imply that these chromosomes became more widely differentiated only late in the therian ancestor, well after the divergence of the monotreme lineage. Thus, our study also provides strong independent support for the recent notion that our sex chromosomes emerged, not in the common ancestor of all mammals, but rather in the therian ancestor, and therefore are much younger than previously thought.
Conflict of interest statement
Figures
References
-
- Emerson JJ, Kaessmann H, Betran E, Long M. Extensive gene traffic on the mammalian X chromosome. Science. 2004;303:537–540. - PubMed
-
- Marques A, Dupanloup I, Vinckenbosch N, Reymond A, Kaessmann H. Emergence of young human genes after a burst of retroposition in primates. PLoS Biol. 2005;3:e357. doi: 10.1371/journal.pbio.0030357. - DOI - PMC - PubMed
-
- Long M, Betran E, Thornton K, Wang W. The origin of new genes: glimpses from the young and old. Nat Rev Genet. 2003;4:865–875. - PubMed
-
- Brosius J. Retroposons—seeds of evolution. Science. 1991;251:753. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

