Evidence of the in vivo esterification of budesonide in human airways
- PMID: 18384442
- PMCID: PMC2485262
- DOI: 10.1111/j.1365-2125.2008.03164.x
Evidence of the in vivo esterification of budesonide in human airways
Abstract
Aims: Budesonide, unlike fluticasone propionate, undergoes fatty acid esterification in the lungs, and there is a need to characterize fully the distribution and fate of the two drugs after inhalation in humans.
Methods: This open-label, randomized study was performed in adults undergoing whole lung or lobar resection resulting from lung cancer. Patients were given single 1000-mug doses of both budesonide and fluticasone propionate via dry powder inhalers before surgery. Tissue samples from peripheral and central lung, an ex vivo bronchial brush sample and intercostal muscle, together with plasma samples, were taken during surgery and analysed by liquid chromatography plus tandem mass spectrometry.
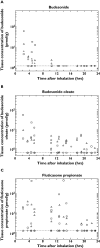
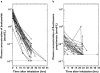
Results: Lung tissue samples were obtained from 22 patients at surgery, 1-43 h after drug dosing. Budesonide was detectable from earliest sampling in central and peripheral lung tissue up to 10 h (in six of 22 samples), fluticasone propionate up to 22 h after inhalation (in 16 of 22 samples), and budesonide oleate up to 43 h after inhalation (in 21 of 22 samples). Budesonide, but not fluticasone propionate, was detected in intercostal muscle for up to 10 h after inhalation. Bronchial brush samples showed the presence of fluticasone propionate for up to 18 h, suggesting the presence of undissolved drug powder particles in the airway lumen.
Conclusion: Sustained retention of esterified budesonide in the lungs supports the prolonged duration of action of budesonide and suitability for once-daily administration.
Figures
Similar articles
-
Nasal retention of budesonide and fluticasone in man: formation of airway mucosal budesonide-esters in vivo.Br J Clin Pharmacol. 2001 Feb;51(2):159-63. doi: 10.1111/j.1365-2125.2001.01303.x. Br J Clin Pharmacol. 2001. PMID: 11259988 Free PMC article. Clinical Trial.
-
Comparison of the systemic effects of fluticasone propionate and budesonide given by dry powder inhaler in healthy and asthmatic subjects.Thorax. 2001 Mar;56(3):186-91. doi: 10.1136/thorax.56.3.186. Thorax. 2001. PMID: 11182010 Free PMC article. Clinical Trial.
-
Plasma concentrations of fluticasone propionate and budesonide following inhalation from dry powder inhalers by healthy and asthmatic subjects.Thorax. 2003 Mar;58(3):258-60. doi: 10.1136/thorax.58.3.258. Thorax. 2003. PMID: 12612308 Free PMC article. Clinical Trial.
-
Inhaled salmeterol/fluticasone propionate combination: a review of its use in persistent asthma.Drugs. 2000 Nov;60(5):1207-33. doi: 10.2165/00003495-200060050-00012. Drugs. 2000. PMID: 11129128 Review.
-
Budesonide fatty-acid esterification: a novel mechanism prolonging binding to airway tissue. Review of available data.Ann Allergy Asthma Immunol. 2002 Jun;88(6):609-16. doi: 10.1016/S1081-1206(10)61893-5. Ann Allergy Asthma Immunol. 2002. PMID: 12086369 Review.
Cited by
-
Development of budesonide microparticles using spray-drying technology for pulmonary administration: design, characterization, in vitro evaluation, and in vivo efficacy study.AAPS PharmSciTech. 2009;10(3):993-1012. doi: 10.1208/s12249-009-9290-6. Epub 2009 Aug 1. AAPS PharmSciTech. 2009. PMID: 19649711 Free PMC article.
-
Budesonide and fluticasone propionate differentially affect the airway epithelial barrier.Respir Res. 2016 Jan 6;17:2. doi: 10.1186/s12931-015-0318-z. Respir Res. 2016. PMID: 26739349 Free PMC article.
-
Effects of budesonide and surfactant in preterm fetal sheep.Am J Physiol Lung Cell Mol Physiol. 2018 Aug 1;315(2):L193-L201. doi: 10.1152/ajplung.00528.2017. Epub 2018 Apr 19. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29671605 Free PMC article.
-
The Modulation of Cell Plasticity by Budesonide: Beyond the Metabolic and Anti-Inflammatory Actions of Glucocorticoids.Pharmaceutics. 2025 Apr 11;17(4):504. doi: 10.3390/pharmaceutics17040504. Pharmaceutics. 2025. PMID: 40284499 Free PMC article. Review.
-
Risks of budesonide/formoterol for the treatment of stable COPD: a meta-analysis.Int J Chron Obstruct Pulmon Dis. 2019 Apr 1;14:757-766. doi: 10.2147/COPD.S192166. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31015757 Free PMC article.
References
-
- National Asthma Education and Prevention Program (NAEPP). Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 1997.
-
- National Asthma Education Prevention Program (NAEPP). Expert Panel Report: guidelines for the diagnosis and management of asthma – update on selected topics. J Allergy Clin Immunol. 2002;110:S141–219. - PubMed
-
- Pauwels R. Use of corticosteroids in asthma. In: D'Arcy PF, McElnay JL, editors. Pharmacy and Pharmacotherapy of Asthma. Chichester: Ellis Harwood; 1989. pp. 104–18.
-
- Edsbäcker S. Pharmacological factors that influence the choice of inhaled corticosteroids. Drugs. 1999;58(Suppl. 4):7–16. - PubMed
-
- Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157:S1–S53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

