A first-in-human phase I trial of locally delivered human plasmin for hemodialysis graft occlusion
- PMID: 18384651
- PMCID: PMC2561322
- DOI: 10.1111/j.1538-7836.2008.02969.x
A first-in-human phase I trial of locally delivered human plasmin for hemodialysis graft occlusion
Abstract
Background: Hemodialysis (HD) grafts often fail because of stenosis at the venous anastomosis and thrombotic occlusion. Percutaneous management relies on thrombolysis with plasminogen activators, mechanical removal of thrombus, and angioplasty of the stenotic lesion.
Objectives: This report describes a phase I trial using Plasmin (Human) TAL 05-00018, a direct-acting fibrinolytic agent, to evaluate safety and, secondarily, to establish effective thrombolytic dosing.
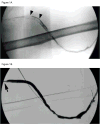
Patients/methods: Six cohorts of five patients with acute HD graft occlusion documented by angiography were treated with escalating dosages of plasmin (1, 2, 4, 8, 12, and 24 mg) infused over 30 min via criss-crossed pulse-spray catheters within the graft. The primary efficacy endpoint was > or =50% thrombolysis, as determined by comparison of pre-plasmin and 30-min post-plasmin fistulograms.
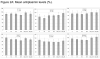
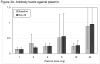
Results: Of 31 subjects who received study drug (safety population), one withdrew and 30 completed the trial (evaluable for efficacy). There was no significant change in plasma alpha-2 antiplasmin or fibrinogen concentration, major bleeding did not occur, and there were no deaths. Serious adverse events in four patients were not related to the study drug. There was a dose-response relationship for the primary efficacy endpoint, all five subjects receiving 24 mg achieving >75% lysis.
Conclusions: This first phase I study of Plasmin (Human) TAL 05-00018, infused into thrombosed HD grafts, documents safety at dosages of 1-24 mg and an effective thrombolytic dosage of 24 mg. The results establish a foundation for further clinical study of catheter-based plasmin administration in thrombotic disorders.
Conflict of interest statement
Figures


References
-
- Marder VJ, Stewart D. Towards safer thrombolytic therapy. Sem Hematol. 2002;39:206–216. - PubMed
-
- Wiman B, Lijnen HR, Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha 2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979;579:142–54. - PubMed
-
- Collen D. On the regulation and control of fibrinolysis. Thromb Haemost. 1980;43:77–89. - PubMed
-
- Marder VJ. The use of thrombolytic agents: Choice of patient, drug administration, laboratory monitoring. Ann Int Med. 1979;90:802–808. - PubMed
-
- Dotter CT, Rosch J, Seaman AJ. Selective clot lysis with low-dose streptokinase. Radiology. 1974;111:31–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

