Numbers needed to treat calculated from responder rates give a better indication of efficacy in osteoarthritis trials than mean pain scores
- PMID: 18384679
- PMCID: PMC2453757
- DOI: 10.1186/ar2394
Numbers needed to treat calculated from responder rates give a better indication of efficacy in osteoarthritis trials than mean pain scores
Abstract
Introduction: Osteoarthritis trials usually report average changes in visual analogue scale (VAS) pain, and examine the difference between treatment and placebo. We investigated whether dichotomous responder analysis provides a more informative interpretation of drug efficacy.
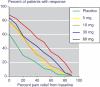
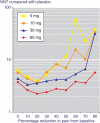
Methods: Merck supplied the number of patients who, by 6 weeks, had achieved pain relief compared with a baseline of 0% or more, 10% or more, 20% or more, and so on at equal intervals up to 90% or more. These different levels of pain relief were used to distinguish different definitions of responders, for example at least 50% pain relief from baseline. Numbers and percentages of patients achieving each level were identified. Information was sought from a dose-response trial over 6 weeks in osteoarthritis using placebo and using etoricoxib at 5, 10, 30 and 60 mg daily.
Results: With placebo, the proportions of patients achieving at least 20%, 50% and 70% pain relief over baseline at 6 weeks were 30%, 11% and 2%. With 60 mg etoricoxib the equivalent percentages were 74%, 49% and 29%. The numbers needed to treat for 30 mg and 60 mg etoricoxib to produce at least 50% pain relief at 6 weeks compared with placebo were 4.2 (95% confidence interval 3.8 to 8.6) and 2.6 (2.0 to 3.9), respectively. Levels of pain relief of 50% and above discriminated best between different doses of etoricoxib.
Conclusion: Responder analysis seemed to be more sensitive than examination of average changes in VAS pain scores. Validation would require calculations to be performed on a set of trials using individual patient data not available in publications.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical