How and why do GPCRs dimerize?
- PMID: 18384890
- PMCID: PMC2652501
- DOI: 10.1016/j.tips.2008.02.004
How and why do GPCRs dimerize?
Abstract
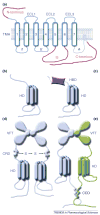
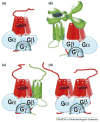
Dimerization is fairly common in the G-protein-coupled receptor (GPCR) superfamily. First attempts to rationalize this phenomenon gave rise to an idea that two receptors in a dimer could be necessary to bind a single molecule of G protein or arrestin. Although GPCRs, G proteins and arrestins were crystallized only in their inactive conformations (in which they do not interact), the structures appeared temptingly compatible with this beautiful model. However, it did not survive the rigors of experimental testing: several recent studies unambiguously demonstrated that one receptor molecule is sufficient to activate a G protein and bind arrestin. Thus, to figure out the biological role of receptor self-association we must focus on other functions of GPCRs at different stages of their functional cycle.
Figures
Similar articles
-
GPCRs and Signal Transducers: Interaction Stoichiometry.Trends Pharmacol Sci. 2018 Jul;39(7):672-684. doi: 10.1016/j.tips.2018.04.002. Epub 2018 May 5. Trends Pharmacol Sci. 2018. PMID: 29739625 Free PMC article. Review.
-
Beta-arrestin signaling and regulation of transcription.J Cell Sci. 2007 Jan 15;120(Pt 2):213-8. doi: 10.1242/jcs.03338. J Cell Sci. 2007. PMID: 17215450 Review.
-
G protein-coupled receptor interactions with arrestins and GPCR kinases: The unresolved issue of signal bias.J Biol Chem. 2022 Sep;298(9):102279. doi: 10.1016/j.jbc.2022.102279. Epub 2022 Jul 19. J Biol Chem. 2022. PMID: 35863432 Free PMC article. Review.
-
Understanding the GPCR biased signaling through G protein and arrestin complex structures.Curr Opin Struct Biol. 2017 Aug;45:150-159. doi: 10.1016/j.sbi.2017.05.004. Epub 2017 May 27. Curr Opin Struct Biol. 2017. PMID: 28558341 Review.
-
To sense or not to sense-new insights from GPCR-based and arrestin-based biosensors.Curr Opin Cell Biol. 2019 Apr;57:16-24. doi: 10.1016/j.ceb.2018.10.005. Epub 2018 Nov 5. Curr Opin Cell Biol. 2019. PMID: 30408632 Review.
Cited by
-
Manipulation of very few receptor discriminator residues greatly enhances receptor specificity of non-visual arrestins.J Biol Chem. 2012 Aug 24;287(35):29495-505. doi: 10.1074/jbc.M112.366674. Epub 2012 Jul 11. J Biol Chem. 2012. PMID: 22787152 Free PMC article.
-
Dimeric arrangement of the parathyroid hormone receptor and a structural mechanism for ligand-induced dissociation.J Biol Chem. 2010 Apr 16;285(16):12435-44. doi: 10.1074/jbc.M109.093138. Epub 2010 Feb 19. J Biol Chem. 2010. PMID: 20172855 Free PMC article.
-
GPCR Allosteric Modulators: Mechanistic Advantages and Therapeutic Applications.Curr Top Med Chem. 2018;18(23):2002-2006. doi: 10.2174/1568026619999190101151837. Curr Top Med Chem. 2018. PMID: 30621563 Free PMC article.
-
Search for Structural Basis of Interactions of Biogenic Amines with Human TAAR1 and TAAR6 Receptors.Int J Mol Sci. 2021 Dec 25;23(1):209. doi: 10.3390/ijms23010209. Int J Mol Sci. 2021. PMID: 35008636 Free PMC article.
-
GPCR: G protein complexes--the fundamental signaling assembly.Amino Acids. 2013 Dec;45(6):1303-14. doi: 10.1007/s00726-013-1593-y. Epub 2013 Sep 20. Amino Acids. 2013. PMID: 24052187 Free PMC article. Review.
References
-
- Rompler H, et al. G protein-coupled time travel: evolutionary aspects of GPCR research. Mol Interv. 2007;7:17–25. - PubMed
-
- Cohen GB, et al. Mechanism of activation and inactivation of opsin: role of Glu113 and Lys296. Biochemistry. 1992;31:12592–12601. - PubMed
-
- Porter JE, et al. Activation of the alpha1b-adrenergic receptor is initiated by disruption of an interhelical salt bridge constraint. J Biol Chem. 1996;271:28318–28323. - PubMed
-
- Ballesteros JA, et al. Activation o f t he beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem. 2001;276:29171–29177. - PubMed
-
- Farrens DL, et al. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

